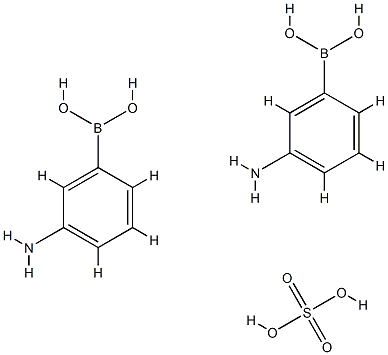
3-Aminobenzeneboronic acid hemisulfate salt
|
|
3-Aminobenzeneboronic acid hemisulfate salt ??
- ???
- ≥300 °C
- ?? ??
- Inert atmosphere,2-8°C
- ???
- ?? ???
- ??? ??
- ??? ??
- ??
- ???? ?? ????
- ??
- Hygroscopic
- BRN
- 8094151
- InChIKey
- XMVGYYBQCXVVHU-UHFFFAOYSA-N
- CAS ??????
- 66472-86-4
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38 | ||
| ????? | 26-36-37/39 | ||
| WGK ?? | 3 | ||
| F ?????? | 3-10 | ||
| ?? ?? | IRRITANT | ||
| HS ?? | 29310095 |
3-Aminobenzeneboronic acid hemisulfate salt C??? ??, ??, ??
??? ??
White to slightly beige crystalline powder??
3-Aminobenzeneboronic acid hemisulfate salt is a boronic acid derivative, which can be used as an intermediate in pharmaceutical synthesis. Adsorbent additive for the chromatographic separation of 3'-terminal polynucleotides from RNA.?? ??
3-Aminophenylboronic acid hemisulfate salt is used in the synthesis of:Phenylboronic acid-functionalized inverse opal hydrogels within microfluidic flow cells for glucose sensing.
Phenylboronic acid-salicylhydroxamic acid-derived complexing reagent for protein immobilization on chromatographic support.
It can also be used in the preparation of phenylboronic acid (PBA) monolayer-modified Au electrode for the voltammetric sensing of uridine and cytidine.
?? ??
3-Aminobenzeneboronic acid hemisulfate salt synthesis: Benzenamine, 3-bromo-N-(diphenylmethylene)- (80,0 g; 0.24 moles) was added to the cryogenic reactor along with 1.4 equivalents of triisopropyl borate and dissolved in tetrahydrofuran. The resulting solution was cooled to -80°C under an inert atmosphere and 1.35 equivalents of n-butyllithium (as a solution in n-hexane) was added maintaining the internal temperature. When complete conversion was achieved, the reaction mixture was added to a dilute mixture of sulfuric acid (1 M) and toluene. The layers were separated to obtain an aqueous solution of 3-aminophenylboronic acid hydrogen sulfate (assay yield: 85%). 3-Aminobenzeneboronic acid hemisulfate salt can be obtained by precipitating a 0.5 M aqueous solution at pH 7.2 (pH corrected with 33% aqueous NaOH) at a temperature of 0-5°C. Filtration and drying under vacuum at 40°C for 8 hours yielded 21.7 g of 3-Aminobenzeneboronic acid hemisulfate salt (0.16 moles; 66% molar yield).3-Aminobenzeneboronic acid hemisulfate salt ?? ?? ? ???
???
?? ??
3-Aminobenzeneboronic acid hemisulfate salt ?? ??
???( 218)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 |
niki@zlchemi.com | China | 3712 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 33025 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29866 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63687 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 |
sales@chemwish.com | CHINA | 10821 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
3-Aminobenzeneboronic acid hemisulfate salt ?? ??:
4-????? ??? ??? ???? 3-????? ??? ??? ???? B-9-??????? ??? ????? ????? ? ????
2-Aminophenylboronic acid
3-AMINOBENZENEBORONIC ACID, PINACOL ESTER HYDROCHLORIDE
4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)aniline hydrochloride
(2-Aminophenyl)boronic acid hydrochloride
4-AMINOPHENYLBORONIC ACID HYDROCHLORIDE
3-AMINOPHENYLBORONIC ACID HYDROCHLORIDE
(2-AMINOPHENYL)BORONIC ACID PINACOL ESTER HYDROCHLORIDE
3-Aminobenzeneboronic acid
3-Aminophenylboronic acid monohydrate
3-Aminobenzeneboronic acid hemisulfate salt
ANILINE SULFATE