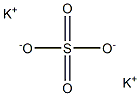
Kaliumsulfat
|
|
Kaliumsulfat Eigenschaften
- Schmelzpunkt:
- 1067°C
- Siedepunkt:
- 1689°C
- chüttdichte
- 800kg/m3
- Dichte
- 2.66
- Flammpunkt:
- 1689°C
- storage temp.
- Inert atmosphere,Room Temperature
- L?slichkeit
- H2O: 0.5 M at 20 °C, clear, colorless
- Aggregatzustand
- Very Fine Crystals or Powder
- Farbe
- White
- Wichte
- 2.662
- Geruch (Odor)
- Odorless
- S?ure-Base-Indikators(pH-Indikatoren)
- ~7
- PH
- 5.5-7.5 (25℃, 0.5M in H2O)
- Biologische Quelle
- synthetic
- Wasserl?slichkeit
- 110 g/L (20 ºC)
- maximale Wellenl?nge (λmax)
- λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01
- Merck
- 14,7674
- crystal system
- Nogata
- Space group
- Pnma
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.7456 0.5776 1.008 90 90 90
- Dielectric constant
- 5.9(0.0℃)
- Stabilit?t:
- Stable.
- InChIKey
- OTYBMLCTZGSZBG-UHFFFAOYSA-L
- CAS Datenbank
- 7778-80-5(CAS DataBase Reference)
- NIST chemische Informationen
- Potassium sulfate(7778-80-5)
- EPA chemische Informationen
- Potassium sulfate (7778-80-5)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| S-S?tze: | 22-24/25 | ||
|---|---|---|---|
| WGK Germany | 1 | ||
| RTECS-Nr. | TT5900000 | ||
| TSCA | Yes | ||
| HS Code | 31043000 | ||
| Giftige Stoffe Daten | 7778-80-5(Hazardous Substances Data) | ||
| Toxizit?t | LD50 orally in Rabbit: 6600 mg/kg |
| Bildanzeige (GHS) |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Kaliumsulfat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS WEISSE KRISTALLE.CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen unter Bildung von Schwefeloxiden.ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).MAK nicht festgelegt (DFG 2005).
INHALATIONSGEFAHREN
Verdampfen bei 20°C vernachl?ssigbar; eine bel?stigende Partikelkonzentration in der Luft kann jedoch beim Dispergieren schnell erreicht werden, besonders als Pulver.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Die Substanz reizt leicht die Augen, die Haut und die Atemwege.
LECKAGE
Verschüttetes Material in Beh?ltern sammeln. Pers?nliche Schutzausrüstung: Atemschutzger?t, P1-Filter für inerte Partikel.S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.S24/25:Berührung mit den Augen und der Haut vermeiden.
Beschreibung
Potassium sulfate (K2SO4) is a kind of chemical compounds that is commonly used in agriculture. The dominant application of potassium sulfate is as a fertilizer, which is commonly applied to offer both potassium and sulfur, thus improving the quality and yield of crops growing in soils that lack an adequate supply of this essential elements. Besides, the crude potassium sulfate is sometimes employed in the production of glass. It also has applications in other industries, which is used as a flash reducer in artillery propellant charges and as an alternative blast media similar to soda in the process of soda blasting.
potassium sulfate powder
Chemische Eigenschaften
Potassium sulfate,K2804, also known as salt of Lemery and arcanite, is a colorless crystalline solid that melts at 1072°C(1960 OF). It is soluble in water,but insoluble in alcohol. Potassium sulfate is used in manufacturing glass, aluminum, fertilizers, and in medicine.Physikalische Eigenschaften
Colorless or white crystals or white granules or powder; rhombohedral structure; bitter taste; density 2.66 g/cm3; melts at 1,069°C; vaporizes at 1,689°C; moderately soluble in water, 12 g/100mL at 25°C and 24g/100mL at 100°C; slightly soluble in glycerol; insoluble in alcohol, acetone, and carbon disulfide.Occurrence
Potassium and sodium sulfates and their double sulfates with calcium and magnesium occur naturally in various salt lakes. Potassium sulfate also occurs in certain volcanic lava. Its double salt with magnesium occurs in nature, as the mineral langbeinite.Potassium sulfate is used in fertilizers as a source of potassium and sulfur, both of which are essential elements for plant growth. Either in sim-ple form or as a double salt with magnesium sulfate, potassium sulfate is one of the most widely consumed potassium salts in agricultural applications. It is preferred over potassium chloride for certain types of crops; such as, tobac-co, citrus, and other chloride-sensitive crops. Some other applications include making gypsum cements; to make potassium alum; in the analysis of Kjeldahl nitrogen; and in medicine.
Verwenden
potassium sulfate is a reagent in cosmetics. Potassium sulfate is an inorganic salt with a primary function as a viscosity-increasing agent.Vorbereitung Methode
Potassium sulfate is produced by various methods, selection of process depending on availability and cost of raw materials.The salt may be obtained from its naturally occurring mineral, langbeinite,K2SO4?2MgSO4. The ore first is crushed and washed with water to separate sodium chloride. After that, magnetite is separated from the washed langbei-nite by magnetic separation. After the separation of these two major impuri-ties, the purified double salt is treated with an aqueous solution of potassium chloride to obtain potassium sulfate:
K2SO4?2MgSO4 + 4KCl →3K2SO4+ 2MgCl2
The solution is filtered to remove insoluble residues and the products are separated from their aqueous mixture by crystallization.
Potassium sulfate also is produced from the mineral kieserite, MgSO4?H2O by treatment with potassium chloride. The intermediate double salt obtained reacts further with potassium chloride to form potassium sulfate:MgSO4?H2O + 2KCl + 4H2O →K2SO4?MgSO4?6H2O + MgCl2
K2SO4?MgSO4?6H2O + 2KCl →2K2SO4+ MgCl2
Potassium sulfate is separated from the more soluble magnesium chloride by crystallization.
Also, potassium sulfate can be made by two other processes in which no naturally occurring mineral is employed. In the Mannheim process, the salt is produced by action of sulfuric acid on potassium chloride:2KCl + H2SO4→K2SO4+ 2HCl
In Hargreaves process, which is a slight variation of the Mannheim method, potassium sulfate is made by heating a mixture of potassium chlo-ride, sulfur dioxide, air and water:4KCl + 2SO2+ 2H2O + o2→2K2SO4+ 4HCl.
Definition
Potassium sulfate is a potassium salt and an inorganic potassium salt. It is a white crystalline powder, K2SO4, soluble inwater and insoluble in ethanol;rhombic or hexagonal; r.d. 2.66; m.p.1069°C. It occurs naturally assch nite (Strassfurt deposits) and inlake brines, from which it is separatedby fractional crystallization. Ithas also been produced by the Hargreavesprocess, which involves theoxidation of potassium chloride withsulphuric acid. In the laboratory itmay be obtained by the reaction ofeither potassium hydroxide or potassiumcarbonate with sulphuric acid.Potassium sulphate is used in cements,in glass manufacture, as a food additive, and as a fertilizer(source of K+) for chloride-sensitiveplants, such as tobacco and citrus.Allgemeine Beschreibung
Potassium sulfate is an inorganic salt that can be prepared by reacting phosphogypsum and potassium chloride. It forms needlelike mullite particles on heating with aluminum sulfate (Al2(SO4)3) and silicon dioxide (SiO2). Its application to the soil has been reported to minimize the bronzing of rice plants. Its surface integration growth kinetics has been obtained in the temperature range of 20-50°C. The theoretical heat capacity curve of potassium sulfate in vapor phase has been obtained.Sicherheitsprofil
Moderately toxic to humans by ingestion. Moderately toxic experimentally by subcutaneous route. Swallowing large doses causes severe gastrointestinal tract effects. When heated to decomposition it emits toxic fumes of K2O and SOx. See also SULFATES.l?uterung methode
Potassium sulfate [7778-80-5] M 174.3, m 1069o, d 4 2.67 It crystallised from distilled water (4mL/g at 20o; 8mL/g at 100o) between 100o and 0o.Einzelnachweise
https://en.wikipedia.org/wiki/Potassium_sulfatehttp://www.cropnutrition.com/potassium-sulfate
http://study.com/academy/lesson/what-is-potassium-sulfate-structure-uses-formula.html
Kaliumsulfat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Kaliumhydroxid
Kaliumcarbonat
Hydrogenchlorid
alunite
Magnesiumsulfat
Kaliumchlorid
Octadecylamin
Ammoniumsulfat
Magnesium sulfate heptahydrate
Schwefelsure
Downstream Produkte
Compound fertilizer
Aluminium potassium sulfate dodecahydrate
ethyl 3-ethylpyrazolyl-5-carboxylate
4-Hydroxybenzoesure
Acenaphthylen
2-Propenal
4-[2-(Diethylamino)ethoxy]benzophenon
Nitrogen phosphorus potassium mixed fertilizer
Dikaliumdisulfat
Nitrogen-Phosphorus-Potassium compound fertilizer with sulphur
Calcium phosphate monobasic
Nicosulfuron
N-1,3-Dimethylbutyl-N'-phenyl-p-phenylendiamin
Kaliumhydrogencarbonat
Mixed and compound fertilizer
POTASSIUM METABISULFITE
Brenztraubensure
Kaliumbromat
Calciumhydrogenorthophosphat
ALPHA-D-GLUCOPYRANOSE 1-PHOSPHATE DIPOTASSIUM SALT HYDRATE
Dikaliumperoxodisulfat
Ammoniumchromat
Compound fertilizer of potassium sulfate
polyhalite
Chrom (III) oxid
Compound fertilizer,high concentration
L-Methionin
Water flush fertilizer
Kaliumsulfat Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 506)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8804 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5887 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +86-15271838296; +8615271838296 |
kyra@quanjinci.com | China | 1512 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 |
admin@hblongbang.com | China | 972 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 299 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 939 | 58 |
7778-80-5(Kaliumsulfat)Verwandte Suche:
Kaliumacrylat
Kaliumacetat
Kaliumchlorid
Potassium sorbate
Kaliumbromat
Losartan potassium
Schwefelsure
Ammoniumsulfat
Cer(4+)disulfat
Hydrazinium(2+)-sulfat
Disilber(1+)sulfat
Magnesiumsulfat
sulfate
Kaliumnitrat
Kaliumcyanid
Dikaliumperoxodisulfat
Aluminiumkaliumbis(sulfat)
Dikaliumdisulfat
- Arcanum duplicatum
- arcanumduplicatum
- dipotassiumsulfate
- Glazier's salt
- kaliumsulphuricum
- Potassium sulfate, alpha
- potassiumsulfate(2:1)
- POTASSIUM SULFATE ANHYDROUS POWDER
- POTASSIUM SULPHATE extrapure AR
- Potassium Sulfate, granular
- Potassium Sulfate, granular(low nitrogen)
- FISHERTAB ST-35 KJELDAHL TABLETS
- FISHERTAB ST-AUTO KJELDAHL TABLETS
- KELMATE(R) KJELDAHL DIGESTION MIXTURE
- KELMATE(R) KJELDAHL DIGESTION MIXTURE CT-37
- KELMATE(R) KJELDAHL DIGESTION MIXTURE MT-37
- KELMATE(R) KJELDAHL DIGESTION MIXTURE ST-35
- KELMATE(R) KJELDAHL DIGESTION MIXTURE ST-AUTO
- KELMATE(R) KJELDAHL DIGESTION MIXTURE TT-35
- KELMATE(R) KJELDAHL DIGESTION MIXTURE TT-50
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 100
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 200
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 210
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 510
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 600
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE NO 610
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 100
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 110
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 200
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 210
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 300
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 410
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 510
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 600
- KELMATE(R) N KJELDAHL DIGESTION MIXTURE PACKETS, NO 610
- KELMATE(R) NT KJELDAHL DIGESTION MIXTURE, NO 710
- KELMATE(R) NT KJELDAHL DIGESTION MIXTURE NO 810
- KJELTAB CATALYSTS
- KJELTABS IB 61
- Potassium sulfate,99+%,for analysis,anhydrous, granular
- Potassium sulfate,99+%,for analysis,anhydrouspowder
- POTASSIUM SULFATE, 99+%, FOR ANALYSIS ACS
- Potassium sulfate, anhydrous, granular, for analysis
- Potassium sulfate, anhydrous powder, for analysis
- Potassium sulfate, for analysis ACS
- Potassium sulfate,agricultural
- POTASSIUM SULFATE POWDER 99+% A.C.S.&
- POTASSIUM SULFATE SIGMAULTRA
- POTASSIUM SULFATE POWDER, PURE
- POTASSIUM SULFATE R. G., REAG. ACS, REAG. ISO, REAG. PH.
- POTASSIUM SULFATE PLANT CELL CULTURE*TES TED
- POTASSIUM SULFATE, ACS
- Potassium Sulfate, Powder, Reagent
- Kalii sulfas
- WATER R PHE
- Potassium sulfate(VI)
- Potassium sulfate, 99.995% trace metals basis
- POTASSIUM SULFATE BIOXTRA