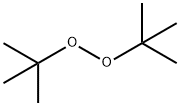
Di-tert-butylperoxid
|
|
Di-tert-butylperoxid Eigenschaften
- Schmelzpunkt:
- -30 °C
- Siedepunkt:
- 109-110 °C(lit.)
- Dichte
- 0.796 g/mL at 25 °C(lit.)
- Dampfdruck
- 40 mm Hg ( 20 °C)
- Brechungsindex
- n
20/D 1.3891(lit.)
- Flammpunkt:
- 34 °F
- storage temp.
- Store at +15°C to +25°C.
- L?slichkeit
- 0.063g/l
- Aggregatzustand
- Liquid
- Farbe
- Clear
- Geruch (Odor)
- distinctive odor
- Wasserl?slichkeit
- immiscible
- Merck
- 14,3461
- BRN
- 1735581
- Stabilit?t:
- May decompose explosively if heated, subjected to shock, or treated with reducing agents. Highly flammable. Refrigerate.
- InChIKey
- LSXWFXONGKSEMY-UHFFFAOYSA-N
- LogP
- 3.2 at 22℃
- CAS Datenbank
- 110-05-4(CAS DataBase Reference)
- NIST chemische Informationen
- Di-tert-butyl peroxide(110-05-4)
- EPA chemische Informationen
- Di-tert-butyl peroxide (110-05-4)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | O,F,Xn | ||
|---|---|---|---|
| R-S?tze: | 7-11-68-52/53-2017/7/11 | ||
| S-S?tze: | 14-16-3/7-36/37/39-14A-61-23 | ||
| RIDADR | UN 3107 5.2 | ||
| WGK Germany | 1 | ||
| RTECS-Nr. | ER2450000 | ||
| Selbstentzündungstemperatur | 182 °C | ||
| HS Code | 2909 60 00 | ||
| HazardClass | 5.2 | ||
| PackingGroup | II | ||
| Giftige Stoffe Daten | 110-05-4(Hazardous Substances Data) | ||
| Toxizit?t | LD50 orally in Rabbit: > 25000 mg/kg |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Di-tert-butylperoxid Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS GELBE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft und k?nnen sich am Boden ausbreiten. Fernzündung m?glich.CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen auf 111°C. Erh?hte Feuergefahr. Starkes Oxidationsmittel. Reagiert sehr heftig mit brennbaren und reduzierenden Stoffen.ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation der D?mpfe.INHALATIONSGEFAHREN
Nur ungenügende Angaben vorhanden über die Geschwindigkeit, mit der eine gesundheitssch?dliche Konzentration in der Luft beim Verdampfen bei 20°C erreicht wird.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Die Substanz reizt die Augen und die Atemwege.
LECKAGE
Belüftung. Zündquellen entfernen. Ausgelaufene Flüssigkeit m?glichst in abdichtbaren Beh?ltern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Kanalisation spülen. NICHT mit S?gemehl oder anderen brennbaren Absorptionsmitteln binden. Pers?nliche Schutzausrüstung: Atemschutzfilter für organische Gase und D?mpfe.R-S?tze Betriebsanweisung:
R7:Kann Brand verursachen.R11:Leichtentzündlich.
S-S?tze Betriebsanweisung:
S14:Von . . . fernhalten (inkompatible Substanzen sind vom Hersteller anzugeben).S16:Von Zündquellen fernhalten - Nicht rauchen.
S3/7:Beh?lter dicht geschlossen halten und an einem kühlen Ort aufbewahren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Beschreibung
Di-tert-butyl peroxide is a clear, water-white liquid. It has a specific gravity of 0.79, which is lighter than water, and it will float on the surface. It is nonpolar and insoluble in water. Di-tert-butyl peroxide is a strong oxidizer and may ignite organic materials or explode if shocked or in contact with reducing agents. In addition to being an oxidizer, Di-tert-butyl peroxide is highly flammable. It has a boiling point of 231°F (110°C) and a flash point of 65°F (18°C). The NFPA 704 designation is health 3, flammability 2, and reactivity 4. The prefix “oxy” for oxidizer is placed in the white section at the bottom of the 704 diamond.Allgemeine Beschreibung
Di-tert-butyl peroxide is a clear colorless liquid. (NTP, 1992)Reaktivit?t anzeigen
The explosive instability of the lower dialkyl peroxides (e.g., dimethyl peroxide) and 1,1-bis-peroxides decreases rapidly with increasing chain length and degree of branching, the di-tert-alkyl derivatives being amongst the most stable class of peroxides. Though many 1,1-bis-peroxides have been reported, few have been purified because of the higher explosion hazards compared with the monofunctional peroxides. Di-tert-butyl peroxide is unlikely that this derivative would be particularly unstable compared to other peroxides in it's class, Bretherick 1979v.Health Hazard
DTBP is slightly toxic by inhalation andin general exhibits low to very low toxicityby other routes. However, toxic effectsare observed only at very high concentrations.Rats exposed to 4103-ppm vapor developedhead and neck tremor after 10 minutesof exposure (Floyd and Stockinger 1958).Other symptoms were weakness, hyperactivity,and labored breathing. However, theanimals recovered fully in 1 hour.LD50 value, intraperitoneal rats): 3210 mg/kg
DTBP is nonirritating to the skin and mildon the eyes. It is reported to cause lungand blood tumors in mice (NIOSH 1986).However, its carcinogenicity is not yet fullyestablished.
Brandgefahr
Highly flammable and reactive; flash point 18°C (64.4°F); vapor pressure 19.5 torr at 20°C (68°F); vapor density 5.03. Its decomposition products are ethane and acetone, which enhance the fire hazard. Use a water spray to fight fire and to keep the containers cool.DTBP forms an explosive mixture with air. The explosive range is not reported. Its decomposition products may explode above its boiling point, 111°C (231.8°F). However, as it is thermally stable and shock insensitive, its explosion hazard is much lower. It may, however, react with explosive violence when in contact with easily oxidizable substances.
Carcinogenicity
A single exposure (route unspecified, but probably subcutaneous (SC)) of 14.6 mg (~365 mg/kg) produced unconvincing evidence for carcinogenicity owing to the lack of controls in 50 mice observed for more than 80 weeks. Of 35 survivors, 7 (20%) had malignant blood tumors (lymphomas) and 1 had a benign lung tumor (pulmonary adenoma) (93, 7a). Owing to its poor design, this study should be judged inadequate to determine carcinogenicity.Lager
Store in a cool and well-ventilated areaisolated from easily oxidizable materials.Protect against physical damage. Shippingcontainers are amber glass and polyethylenebottles or steel drums not exceeding 100-lbcapacity.l?uterung methode
Wash the peroxide with aqueous AgNO3 to remove olefinic impurities, water and dry (MgSO4). Free it from tert-butyl hydroperoxide by passage through an alumina column [Jackson et al. J Am Chem Soc 107 208 1985], and if necessary two high vacuum distillations from room temperature to a liquid-air trap [Offenbach & Tobolsky J Am Chem Soc 79 278 1957]. [Beilstein 1 IV 1619.] The necessary protection from EXPLOSION should be used.Waste disposal
Di-tert-butyl peroxide is disposed on the ground in a remotearea and ignited with a long torch. 10%NaOH may be used to wash empty containers.Di-tert-butylperoxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
(4-METHOXY-PYRIDIN-2-YL)-METHANOL
4-METHOXYPICOLINALDEHYDE
CH3
4-Fluoro-2-methylbenzoic acid
[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER
4-trans-Propenylveratrol
N-(TERT-BUTOXYCARBONYL)-3-BROMOANILINE
TERT-BUTYL 4-CYANOPHENYLCARBAMATE
Methyl-2-nitrobenzoat
1-Methylcaffeine
4-bromo-2,6-dimethylbenzoic acid
4-Methyl-benzoic acid tert-butyl ester
1-Fluor-4-(methylthio)benzol
3-Bromothioanisole
2-Methylthiopyridine
Trimethyloxiran
1-Brom-4-(methylsulfonyl)benzol
Di-tert-butylperoxid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 293)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Sinomore New Material Company Limited | +86-13656229759 +86-13656229759 |
sales@sinomorechem.com | China | 25 | 58 |
| Wuxi High Mountain Hi-tech Development Co.,Ltd. | +86-86-0510-85881806 +8613357920996 |
wuxihighmountain@gmail.com | China | 59 | 58 |
| TEKA-FAST LIMITED | +8618601192704 |
info@tekafast.com | China | 76 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12830 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5856 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 8753 | 55 |
| Chemson Industrial (Shanghai) Co., Ltd. | 86-21-65208861- 8007 |
sales1@chemson.com.cn | CHINA | 117 | 58 |
| Tianjin Zhongxin Chemtech Co., Ltd. | 86-22-66880623 +8618622897568 |
sales@tjzxchem.com | China | 569 | 58 |
110-05-4(Di-tert-butylperoxid)Verwandte Suche:
tert-Butylperacetat
tert-Butylhydroperoxid
tert-Butylchlordimethylsilan
Dibenzoylperoxid
Dilauroylperoxid
4-tert-Butylphenol
(tert-Butyl)methylether
Peroxidase
Methyl-4-tert-butylbenzoat
2-Methylpropanol-2
2,6-Di-tert-butylphenol
2,6-Di-tert-butyl-p-kresol
Peroxidase, Glutathion-
Peroxidase(POD)
2,4-Di-tert-butylphenol
tert-Butylperbenzoat
4-(1,1-Dimethylethyl)-1,2-benzoldiol
tert-Butylacetat
- (tert-C4H9O)2
- Aztec di-t-butyl peroxoide
- bis(1,1-dimethylethyl)-peroxid
- bis(t-butyl)peroxide
- Bis(tert-butyl) peroxide
- Cadox
- Cadox tbp
- cadoxtbp
- dibutylperoxide(non-specificname)
- Di-tert-butyl peroxyde
- Di-tert-butylperoxid
- di-tert-butylperoxyde
- interoxdtb
- kayabutyld
- perbutyld
- Perossido di butile terziario
- perossidodibutileterziario
- Peroxide, bis(1,1-dimethylethyl)
- Peroxide, tert-butyl-
- Peroxide,bis(1,1-dimethylethyl)
- Peroxyde de butyle tertiaire
- peroxydedebutyletertiaire
- peroxydedebutyletertiaire(french)
- t-butyl peroxide bis(1,1-di-methylethyl)peroxide
- tert-Dibutyl peroxide
- tertiary-butylperoxide
- trigonoxb
- tert-Butyl peroxide (Luperox®
- Luperox(R) DI, tert-Butyl peroxide 98%
- T-BUTYL PEROXIDE
- Luperox? DI, tert-Butyl peroxide
- Di-tert-butyl peroxide
- Di-tert-butyl peroxide (tert-Butyl peroxide)
- Di-#ntert.-butyl peroxide
- DIBUTYLPEROXIDE
- DI-TERT-BUTYL PEROXIDE
- BIS(1,1-DIMETHYLETHYL)PEROXIDE
- tert-Butyl peroxide (Luperox(R) DI)
- tert-Butyl peroxide, Di-tert-butyl peroxide
- Initiating agent A
- Di-tert-B-butyl peroxide, 99%
- Di-tert-butyl peroxide,99%
- di-t-butyl peroxoide
- tert-Butyl peroxide (Luperox DI)
- tert-Butyl peroxide (Luperox DI),tert-Butyl peroxide, Di-tert-butyl peroxide
- Di-tert-butyl peroxi
- LUPEROX(R) DI
- Di-tert-butylPeroxide>
- DI-TERT-BUTYL PEROXIDE FOR SYNTHESIS
- Luperox(R) DI, tert-Butyl peroxide
- Di-tert-butyl peroxide ISO 9001:2015 REACH
- (tributyl)peroxide
- 2-(tert-Butylperoxy)-2-methylpropane
- bis(tert-butyl)peroxide
- Trigonox b
- TERT-BUTYL PEROXIDE
- DI-T-BUTYL PEROXIDE
- DI-TERTIARY-BUTYL PEROXIDE