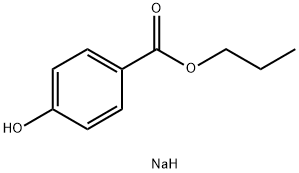
Natrium-4-propoxycarbonylphenoxid
|
|
Natrium-4-propoxycarbonylphenoxid Eigenschaften
- Schmelzpunkt:
- 302°C
- Dichte
- 1.24 g/cm3 (25 °C)
- Dampfdruck
- 0.001Pa at 20℃
- storage temp.
- Store at +15°C to +25°C.
- L?slichkeit
- Freely soluble in water, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
- pka
- 8.46[at 20 ℃]
- Aggregatzustand
- Solid
- Farbe
- White to Off-White
- PH
- 9.5-10.5 (1g/l, H2O, 20°C)
- Wasserl?slichkeit
- 1000g/L at 23℃
- Stabilit?t:
- Hygroscopic
- LogP
- 0.27 at 23℃
- CAS Datenbank
- 35285-69-9(CAS DataBase Reference)
- EPA chemische Informationen
- Propylparaben sodium (35285-69-9)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| S-S?tze: | 22-24/25 | ||
|---|---|---|---|
| WGK Germany | 2 | ||
| RTECS-Nr. | DH2850000 | ||
| HS Code | 2918 29 00 | ||
| Toxizit?t | LD50 orally in Rabbit: > 5000 mg/kg |
| Bildanzeige (GHS) |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Natrium-4-propoxycarbonylphenoxid Chemische Eigenschaften,Einsatz,Produktion Methoden
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.S24/25:Berührung mit den Augen und der Haut vermeiden.
Chemische Eigenschaften
White or almost white, hygroscopic, crystalline powder.Verwenden
Sodium Propylparaben is a preservative. Sodium Propylparaben is also an excipient used in various pharmaceutical formulations.Vorbereitung Methode
Propylparaben sodium is produced from benzoic acid.Pharmazeutische Anwendungen
Propylparaben sodium is used as an antimicrobial or antifungal preservative in oral pharmaceuticals and in many water-based cosmetics. It is generally used in combination with other paraben esters.Kontakt-Allergie
This substance is one of the parabens family. Parabens are esters formed by p-hydroxybenzoic acid and an alcohol. They are largely used as biocides in cosmetics and toiletries, medicaments, or food. They have synergistic power with other biocides. Parabens can induce allergic contact dermatitis, mainly in chronic dermatitis and wounded skin.Sicherheit(Safety)
Propylparaben sodium is used in oral pharmaceuticals and cosmetics. The pure form is toxic by the IV route and moderately toxic by ingestion and the IP route. Propylparaben sodium may cause asthma, rashes, and hyperactivity.LD50 (mouse, IP): 0.49 g/kg
LD50 (mouse, IV): 0.18 g/kg
LD50 (mouse, oral): 3.7 g/kg
Lager
Propylparaben sodium is stable under normal conditions. It decomposes on heating. Store in a tightly closed container.Inkompatibilit?ten
The activity of propylparaben sodium can be adversely affected by the presence of other excipients or active ingredients, such as atropine, essential oils, iron, magnesium trisilicate, talc, polysorbate 80 and other nonionic surfactants, sorbitol, weak alkalis, and strong acids.Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules, tablets, suspensions). Accepted for use as a food additive in Europe. Included in nonparenteral medicines (oral capsules, mixtures, orodispersible tablets, solutions and suspensions; cutaneous emulsions) licensed in the UK.Natrium-4-propoxycarbonylphenoxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Natrium-4-propoxycarbonylphenoxid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 426)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 193 | 55 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12831 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 80 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3007 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 |
admin@juchuangchem.com | China | 298 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +8613288715578 |
angelia@hbmojin.com | China | 1175 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18940 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
35285-69-9(Natrium-4-propoxycarbonylphenoxid)Verwandte Suche:
Natrium-4-(methoxycarbonyl)phenolat
Natrium-4-ethoxycarbonylphenoxid
Ethyl-4-hydroxybenzoat
Propyl-4-hydroxybenzoat
Ethyl-4-cyanbenzoat
Methyl-4-cyanbenzoat
4-Hydroxyzimtsure
Methyl-4-hydroxyphenylacetat
Arginin
Methylhydrogenfumarat
Methyl-4-hydroxybenzoat
Butyl-4-hydroxybenzoat
4-Hydroxybenzoesure
Natriumphenoxid
Natriumbutyl-4-hydroxybenzoat
Natrium-4-propoxycarbonylphenoxid
Natrium-p-kresolat
- SODIUM PROPYL 4-HYDROXYBENZOATE
- Sodium propylparaben
- SolbrolP,Natriumsalz
- PROPYLPARABENSODIUM,NF
- PROPYL4-HYDROXYBENZOATESODIUMSALT(PROPYLPARABENSODIUMSALT)
- Sodiumpropylester4-hydroxybenzoate
- SOIDUM PROPYLPARABEN
- 4-hydroxybenzoic acid propyl ester sodium salt
- PROPYL 4-HYDROXYBENZOATE SODIUM SALT, PH EUR
- Propyl-4-HydroxybenzoateSodiumSaltPurified
- PropylParabenSodiumPropylParabenSodiumBp/Usp/Ep
- PROPYL-4-HYDROXY BENZOATE SODIUM SALT PURE
- 4-(Sodiooxy)benzoic acid propyl ester
- p-Sodiooxybenzoic acid propyl ester
- D02459
- Propylis parahydroxybenzoas natricum
- Propyl 4-hydroxybenzoate sodium salt,Propylis parahydroxybenzoas natricum, Sodium propyl 4-hydroxybenzoate
- Propyl 4-Hydroxybenzoate SodiuM Salt, BP, NF, Ph Eur
- propylparabens
- Propylis parahydroxybenzoas natricum, Sodium propyl 4-hydroxybenzoate
- PROPYL PARABEN SODIUM SALT
- PROPYL P-HYDROXYBENZOATE SODIUM SALT
- PROPYL 4-HYDROXYBENZOATE SODIUM
- NIPASOL SODIUM
- benzoicacid,4-hydroxy-,propylester,sodiumsalt
- Benzoicacid,p-hydroxy-,propylester,sodiumderiv.
- Paradept
- propil-p-hydroxybenzoate,sodiumsalt
- sodium4-propoxycarbonylphenoxide
- 4-Hydroxybenzoic acid propyl ester sodiuM
- Sodium 4-(propoxycarbonyl)
- PROPYL PARABEN SODIUM BP (SODIUM PROPYL
- SodiuM proply p-hydroxybenzoate
- Benzoic acid,4-hydroxy-, propyl ester, sodiuM salt (1:1)
- p-Hydroxybenzoic acid propyl ester sodium salt
- Sodium Propyl-p-hydroxyl Benzoate
- 4-Hydroxybenzoic acid propyl ester sodium salt/Sodium propylparaben
- Sodium Propylparaben,>98%
- Methylparaben Impurity 2 Sodium Salt(Propylparaben Sodium Salt)
- Propyl 4-hydroxybenzoate sodium salt tested according to Ph.Eur.
- Sodium Propyl Paraben IP/BP/USP/Ph.Eur
- Food Grade Preservative Sodium Propylparaben CAS No.: 35285-69-9
- Propyl-4-Hydroxybenzoate Sodium Salt (Propyl Paraben Sodium) ExiPlus, Multi-Compendial, 94-102%
- PROPYL-4-HYDROXYBENZOATE SODIUM SALT Extra Pure
- SODIUM PROPYL HYDROXYBENZOATE
- SODIUM PROPYL-P-HYDROXYBENZOATE
- PROPYLPARABEN SODIUM
- PROPYL 4-HYDROXYBENZOATE SODIUM SALT
- SodiuM 4-(propoxycarbonyl)phenolate
- propyl4-hydroxyben zoate sodiu salt
- Propyl 4-hydroxybenzoate sodium salt Ph
- SODIUM PROPYLPARABEN IH
- 35285-69-9
- 5285-69-9
- 35285-69-6
- C10H11O3Na
- C10H11NaO3
- API