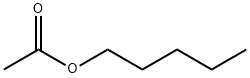
Pentylacetat
|
|
Pentylacetat Eigenschaften
- Schmelzpunkt:
- −100 °C(lit.)
- Siedepunkt:
- 142-149 °C(lit.)
- Dichte
- 0.876 g/mL at 25 °C(lit.)
- Dampfdichte
- 4.5 (vs air)
- Dampfdruck
- 4 mm Hg ( 20 °C)
- Brechungsindex
- n
20/D 1.402(lit.)
- Flammpunkt:
- 75 °F
- storage temp.
- Store below +30°C.
- L?slichkeit
- 10g/l
- Aggregatzustand
- Powder
- Farbe
- White
- Geruch (Odor)
- Pleasant banana-like; mild; characteristic banana- or pear-like odor.
- Explosionsgrenze
- 1.1-7.5%(V)
- Biologische Quelle
- synthetic
- Geruchsart
- fruity
- Wasserl?slichkeit
- 10 g/L (20 ºC)
- FreezingPoint
- -70.8℃
- BRN
- 1744753
- Henry's Law Constant
- 10.73 at 37 °C (static headspace-GC, Bylaite et al., 2004)
- Expositionsgrenzwerte
- TLV-TWA 100 ppm (~525 mg/m3) (ACGIH, MSHA, and OSHA); IDLH 4000 ppm.
- Dielectric constant
- 5.0(20℃)
- LogP
- 2.300
- Oberfl?chenspannung
- 25.8mN/m at 298.15K
- CAS Datenbank
- 628-63-7(CAS DataBase Reference)
- NIST chemische Informationen
- Acetic acid, pentyl ester(628-63-7)
- EPA chemische Informationen
- n-Amyl acetate (628-63-7)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xi | ||
|---|---|---|---|
| R-S?tze: | 10-66 | ||
| S-S?tze: | 23-25-24/25 | ||
| RIDADR | UN 1104 3/PG 3 | ||
| OEB | A | ||
| OEL | TWA: 100 ppm (525 mg/m3) | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | AJ1925000 | ||
| F | 21 | ||
| Selbstentzündungstemperatur | 680 °F &_& 714 °F | ||
| TSCA | Yes | ||
| HazardClass | 3 | ||
| PackingGroup | III | ||
| HS Code | 29153930 | ||
| Giftige Stoffe Daten | 628-63-7(Hazardous Substances Data) | ||
| Toxizit?t | Acute oral LD50 for rats 6,500 mg/kg (quoted, RTECS, 1985). | ||
| IDLA | 1,000 ppm |
| Bildanzeige (GHS) |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Pentylacetat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft.CHEMISCHE GEFAHREN
Reagiert mit Oxidationsmitteln unter Feuer- und Explosionsgefahr. Greift viele Kunststoffe an.ARBEITSPLATZGRENZWERTE
TLV: 50 ppm (als TWA); 100 ppm (als STEL); (ACGIH 2005).MAK: 50 ppm, 270 mg/m? Spitzenbegrenzung: überschreitungsfaktor I(1); Schwangerschaft: Gruppe C; (DFG 2008).
EG Arbeitsplatz-Richtgrenzwerte: 50 ppm, 270 mg/m?(als TWA); 100 ppm, 540 mg/m?(als STEL) (EU 2000).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation der D?mpfe.INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitssch?dliche Kontamination der Luft ein.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Die Substanz reizt die Augen, die Haut und die Atemwege. Exposition in hohen Konzentrationen kann zu Bewusstseinstrübung führen.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Die Flüssigkeit entfettet die Haut.LECKAGE
Zündquellen entfernen. Ausgelaufene Flüssigkeit m?glichst in abdichtbaren Beh?ltern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen.R-S?tze Betriebsanweisung:
R10:Entzündlich.R66:Wiederholter Kontakt kann zu spr?der oder rissiger Haut führen.
S-S?tze Betriebsanweisung:
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).S25:Berührung mit den Augen vermeiden.
S24/25:Berührung mit den Augen und der Haut vermeiden.
Chemische Eigenschaften
All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.Physikalische Eigenschaften
Colorless liquid with a sweet, banana-like odor. A detection odor threshold concentration of 275 μg/m3 (52 ppbv) was reported by Punter (1983). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 1,650 ppmv.Verwenden
A colorless liquid made by adding sulfuric acid to a mixture of amyl alcohol and acetic acid with subsequent recovery by distillation. It is slightly soluble in water but insoluble in alcohol. Amyl acetate was used as one of the solvents in making celluloid film and as fuel for the Alteneck lamp, adopted as the standard light in sensitometry by the International Congress of Photography in 1889.Vorbereitung Methode
n-Amyl acetate is the produced by the esterification of N-amyl alcohol with acetic acid.Definition
ChEBI: An acetate ester of pentanol.Allgemeine Beschreibung
A mixture of isomers. A clear colorless liquid with a banana-like odor. Flash point varies from 65°F. to 95°F. Less dense (at 7.2 lb / gal) than water and slightly soluble in water. Hence floats on water. Vapors heavier than air.Air & Water Reaktionen
Highly flammable. Slightly soluble in water.Reaktivit?t anzeigen
AMYL ACETATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Amyl acetate is incompatible with the following: Nitrates; strong oxidizers, alkalis & acids .Hazard
Flammable, high fire risk. Explosive limits in air 1.1–7.5%.Health Hazard
n-Amyl acetate is a narcotic, an irritant tothe eyes and respiratory passage, and at highconcentrations, an anesthesia. Exposure toabout 300 ppm in air for 30 minutes mayproduce eye irritation in humans. Higherconcentrations (>1000 ppm) may produceheadache, somnolence, and narcotic effects.Exposure to 5200 ppm for 8 hours was lethalto rats. It is more toxic than the loweraliphatic esters. An LD50 value in rats iswithin the range 6000 mg/kg.Brandgefahr
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Chemische Reaktivit?t
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Sicherheitsprofil
Moderately toxic by intraperitoneal route. Human systemic effects by inhalation: conjunctiva irritation, headache, and somnolence. A human eye irritant. Apparently more toxic than butyl acetate. Chronic toxicity is of a low order. Dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. Moderately explosive in the form of vapor when exposed to flame. To fight fire, use alcohol foam, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS, AMYL ALCOHOL, and ACETIC ACID.m?gliche Exposition
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.Environmental Fate
Chemical/Physical. Hydrolyzes in water forming acetic acid and 1-pentanol.At an influent concentration of 985 mg/L, treatment with GAC resulted in an effluent concentration of 119 mg/L. The adsorbability of the carbon used was 175 mg/g carbon (Guisti et al., 1974).
Versand/Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.l?uterung methode
Shake the ester with saturated NaHCO3 solution until neutral, washed it with water, dry with MgSO4 and distil it. The ester has also been purfied by repeated fractional distillation through an efficient column or spinning band column. [Timmermann & Hennant-Roland J Chim Phys 52 223 1955, Mumford & Phillips J Chem Soc 75 1950, 1H NMR: Crawford & Foster Can J Phys 34 653 1956, Beilstein 2 IV 152.]Inkompatibilit?ten
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.Waste disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Pentylacetat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Pentylacetat Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 424)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 |
abby@chuanghaibio.com | China | 8809 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12818 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5855 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5876 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 |
sales@qdrenas.com | China | 587 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21631 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29863 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
628-63-7(Pentylacetat)Verwandte Suche:
Phenylacetat
POLY(VINYL ACETATE)
Natriumacetat
Triacetin
Pentylsalicylat
2-Methylbutylacetat
CELLULOSE ACETATE
Isopentylnitrit
Isopentylacetat
Linalyl acetate
2-Ethoxy-ethylacetat
Vinylacetat
2-Butoxy-ethylacetat
(1α,2β,5α)-2-Methyl-5-(1-methylvinyl)cyclohexylacetat
Ethylacetat
Glatiramer acetate
2-Methoxy-1-methylethylacetat
sec-Butylacetat
- PENTANYL ACETATE
- PEAR OIL
- n-Pentylacetate,99%
- n-Amyl acetate, 99+%
- 1-Acetoxypentane
- 1-Pentanol acetate
- 1-pentanol,acetate
- 1-pentanolacetate
- Acetate d'amyle
- acetated’amyle
- acetated’amyle(french)
- acetated’amylenormal
- Acetic acid, amyl ester
- AMYL ACETATE 99+%
- AmylAcetate,Certified
- Pentylacetate,tech.
- AMYLACETATE,NATURAL
- AMYLACETATE,REAGENT
- AMYLACETATE,TECHNICAL
- AMYL ACETATE(SG)
- Pentyl acetate (all isomers)
- Amyl acetate, Pentyl acetate
- prim-Pentyl acetate
- Acetic acid, 1-pentyl ester
- Acetic acid amyl
- Acetic acid pentyl
- n-Pentyl acetate, tech.
- Acetic Acid Amyl Ester Pentyl Acetate
- Amyl acetate, mixture of isomers, synthesis grade
- Amyl acetate,Amyl acetate, Pentyl acetate
- n-AMyl acetate, 99% 250ML
- Essigsure-n-amylester
- amyl acetate >=99.0%
- Acetic acid, n-pentyl ester
- aceticacid,amylester
- Aceticacidamylester
- ai3-02729
- Amyl acetic ether
- Amylacetateester
- amylacetates
- amylaceticether
- Amylazetat
- Amylester kyseliny octove
- amylesterkyselinyoctove
- amylesterkyselinyoctove(czech)
- Birnenoel
- caswellno049a
- dymonswhwasp&hornetspray
- epapesticidechemicalcode000169
- Ethanoicacidpentylester
- holidaypetrepellent
- holidayrepellentdust
- n-Amylacetat
- n-amylacetate,technicalgrade
- normal
- normalamylacetate
- n-Pentyl ethanoate
- Octan amylu