Sertaconazole nitrate
|
|
|
- CAS-Nr.
- 99592-32-2
- Englisch Name:
- Sertaconazole nitrate
- Synonyma:
- SERTACONAZOLE;eno;Zalain;FI-7056;fi-7045;Dermofix;Sertaconazol;Sertaconazole API;Sertaconazole nitrate;Sertaconazole, 10 mM in DMSO
- CBNumber:
- CB6701774
- Summenformel:
- C20H15Cl3N2OS
- Molgewicht:
- 437.77
- MOL-Datei:
- 99592-32-2.mol
|
Sertaconazole nitrate Eigenschaften
- Schmelzpunkt:
- 146-147°
- Siedepunkt:
- 614.1±55.0 °C(Predicted)
- Dichte
- 1.43±0.1 g/cm3(Predicted)
- storage temp.
- Refrigerator
- L?slichkeit
- DMSO (Slightly), Methanol (Slightly)
- Aggregatzustand
- Solid
- pka
- 6.68±0.12(Predicted)
- Farbe
- White to Pale Yellow
- CAS Datenbank
- 99592-32-2(CAS DataBase Reference)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H315 |
Verursacht Hautreizungen. |
Hautreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P302+P352, P321,P332+P313, P362 |
| H319 |
Verursacht schwere Augenreizung. |
Schwere Augenreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P305+P351+P338,P337+P313P |
| H335 |
Kann die Atemwege reizen. |
Spezifische Zielorgan-Toxizit?t (einmalige Exposition) |
Kategorie 3 (Atemwegsreizung) |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
|
|
| Sicherheit |
| P264 |
Nach Gebrauch gründlich waschen. |
| P264 |
Nach Gebrauch gründlich waschen. |
| P280 |
Schutzhandschuhe/Schutzkleidung/Augenschutz tragen. |
| P302+P352 |
BEI BERüHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckm??ig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen. |
| P305+P351+P338 |
BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach M?glichkeit entfernen. Weiter spülen. |
| P321 |
Besondere Behandlung |
| P332+P313 |
Bei Hautreizung: ?rztlichen Rat einholen/?rztliche Hilfe hinzuziehen. |
| P362 |
Kontaminierte Kleidung ausziehen und vor erneutem Tragen waschen. |
|
Sertaconazole nitrate Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Sertaconazole has been developed and launched for the
treatment of dermatological fungal infections by Ferrer
Internacional S. A. Mylan received FDA approval for
sertaconazole nitrate cream for the treatment of athlete's foot
(tinea pedis) at the end of 2003.
Verwenden
An imidazole antifungal agent, inhibits the synthesis of ergosterol, an essential cell wall component of fungi.
Synthese
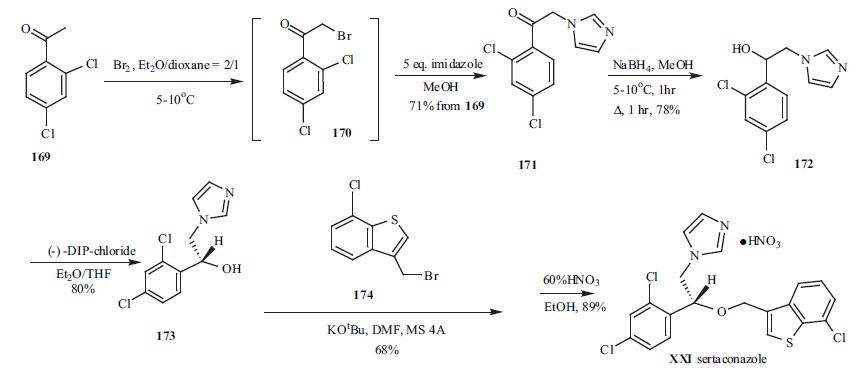
2,4-Dichloro acetophenone
169 was brominated at low temperature to give bromide
intermediate 170, which was used without isolation. To the
same pot, five-fold excess of imidazole was added to give
imidazolylacetophenone 171 in 71% yield from 169.
Sodium borohydride was employed to reduce ketone 171 to
alcohol 172 in 78% yield. Racemic alcohol 172 was resolved with (-)-DIP-chloride to give its corresponding
chiral R-alcohol 173 in 80% yield. Compound 173 was then
alkylated with 3-bromomethyl-7-chlorobenzo[b]thiophene
(174) in dry DMF in the presence of potassium t-butoxide to
give the alkylation product in 68% yield. Finally, 60%
nitric acid was used to make sertaconazole mononitrate
(XXI) in 89% yield.
Solubility in organics
Fairly soluble in ethanol (1.7 %), chloroform (1.5 %); slightly soluble in acetone (0.95 %); very slightly soluble in noctanol (0.069 %). Practically insoluble in water (< 0.01 %).
Sertaconazole nitrate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Sertaconazole nitrate Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 141)Lieferanten
99592-32-2()Verwandte Suche:
- 1-(2-((7-chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-1h-i
- Dermofix
- FI-7056
- Zalain
- 1-[2-[(7-Chlorobenzo[b]thiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole
- 1-[β-[(7-Chlorobenzo[b]thiophene-3-yl)methoxy]-2,4-dichlorophenethyl]-1H-imidazole
- Sertaconazol
- (+-)-1-(2,4-Dichloro-beta-((7-chlorobenzo(b)thien-3-yl)methoxy)phenethyl)imidazole
- 1h-imidazole,1-(2-((7-chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)
- 7-chloro-3-(1-(2,4-dichlorophenyl)-2-(1h-imidazol-1-yl)ethoxy-methyl)benzo(b
- 7-cloro-3-(1-(2,4-diclorofenil)-2-(1h-imidazol-1-il)etoxi-metil)benzo(b)tiof
- fi-7045
- 7-CHLORO-3-[1-(2,4-DICHLOROPHENYL)-2-(1H-IMIDAZOL-1-YL)ETHOXY-METHYL] BENZO[B]THIOPHENE NITRATE
- 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole nitrate
- 1-[2-[(7-Chlorobenzo[b]thien-3-y1)methoxy]-2-(2����,4-dichlorophenyl)ethyl]-1H-imidazole
- Sertaconazole nitrate
- 1H-Imidazole, 1-[2-[(7-chlorobenzo[b]thien-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-
- Sertaconazole nitrate USP/EP/BP
- Sertaconazole API
- eno
- SERTACONAZOLE
- (RS)-1-(2-((7-chloro-1-benzothiophen-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl) -1H-imidazole
- Sertaconazole, 10 mM in DMSO
- 99592-32-2
- C20H15Cl3N2OSHNO3
- C20H15N2OCl3S
- Aromatics
- Heterocycles
- Intermediates & Fine Chemicals
- Pharmaceuticals
- Sulfur & Selenium Compounds

