N,N'-Methantetraylbis(1-methylethylamin) Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R10:Entzündlich.
R26:Sehr giftig beim Einatmen.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R41:Gefahr ernster Augensch?den.
R42/43:Sensibilisierung durch Einatmen und Hautkontakt m?glich.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S38:Bei unzureichender Belüftung Atemschutzger?t anlegen.
S16:Von Zündquellen fernhalten - Nicht rauchen.
Beschreibung
Diisopropylcarbodiimide (DIC) is a clear liquid that can be easily dispensed by volume. It slowly reacts with moisture from the air, so for long term storage the bottle should be flushed with dry air or inert gas and sealed tightly. It is used in peptide chemistry as a coupling reagent. It is very toxic and caused contact dermatitis in a laboratory worker.
Chemische Eigenschaften
N,N'-Diisopropylcarbodiimide is colorless to pale yellow liquid. Insoluble in water, soluble in benzene, ethanol, ether.
Verwenden
N,N'-Diisopropylcarbodiimide is used as a reagent in synthetic organic chemistry. It serves as a chemical intermediate and as a stabilizer for Sarin (chemical weapon). It is also used in the synthesis of peptide and nucleic acid. Further, it is used as an antineoplastic and involved in the treatment of malignant melanoma and sarcomas. It is mainly used in amikacin, glutathione dehydrants, as well as in synthesis of acid anhydride, aldehyde, ketone, isocyanate; when it is used as dehydrating condensing agent, it reacts to dicyclohexylurea through short-time reaction under normal temperature.
Definition
ChEBI: A carbodiimide compound having an isopropyl substituent on both nitrogen atoms.
Allgemeine Beschreibung
N,N′-Diisopropylcarbodiimide (DIC) is a carbodiimide used as a coupling reagent in the synthesis of amides, peptides, ureas, heterocycles, and unsymmetrical carbodiimides. It is also used in the polymerization reactions as an activator.
Kontakt-Allergie
It is used in peptide chemistry as a coupling reagent. It
is very toxic and causes contact dermatitis in labora-
tory workers.
Synthese
Add N, N'diisopropylthiourea to the autoclave and use xylene as the solvent. The mass ratio of N, N'diisopropylthiourea to xylene solvent is 2:1. Add Sb2O4 catalyst (the amount of the catalyst is 1% of the total material), and oxygen is introduced. The temperature is increased to 115°C under a pressure of 8MPa for 4h, then lowered. Suction filtration at 15°C, decolorization, and vacuum distillation were used to obtain N, N'-Diisopropylcarbodiimide. The yield is 94.55%, and the purity is 99.55%.
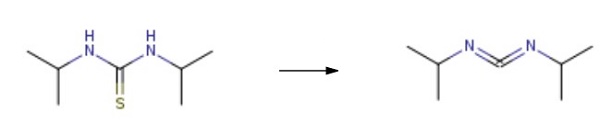
N,N'-Methantetraylbis(1-methylethylamin) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
4-(PIPERIDINE-1-CARBONYL)PHENYLBORONIC ACID
4-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID
3-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID
4-(3-TERT-BUTYL-1,2,4-OXADIAZOL-5-YL)PHENYLBORONIC ACID
3-TERT-BUTYL-5-(4-BROMOPHENYL)-1,2,4-OXADIAZOLE
4-(3-TERT-BUTYL-1,2,4-OXADIAZOL-5-YL)BENZALDEHYDE
2-TERT-BUTYL-1,3-DIISOPROPYLISOUREA
4,5-Imidazolidinedione, 2,2-dichloro-1,3-bis(1-methylethyl)-
BIS(N N'-DIISOPROPYLACETAMIDINATO)NICKE&

