Beryllium hydride Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
BeH2 was first synthesized in 1951 by reacting dimethylberyllium,
Be(CH3)2, with lithium aluminum
hydride, LiAlH4. A purer grade of BeH2 can also be
formed from the pyrolysis of di-tert-butylberyllium,
{Be(C(CH3)3)2} at 210°C. The purest beryllium hydride
is obtained by the reaction of triphenylphosphine, i.e.
PPh3,with berylliumborohydride,Be(BH4)2 by the reaction:
Be(BH4)2+2PPh3→2Ph3PBH3 + BeH2
Note that unlike the other elements in Group IIA
where the hydride can be prepared by reaction of the
elements, the reaction of the metal with hydrogen to
produce beryllium hydride has not proved possible.
BeH2 is usually formed as an amorphous white solid,
but a hexagonal crystalline form with a higher density
(~0.78 g/cm3) has also been reported. This was prepared
by heating amorphous BeH2 under pressure, with
0.5–2.5% LiH as a catalyst. A more recent investigation
found that crystalline beryllium hydride has a bodycentered
orthorhombic unit cell, containing a network
of corner-sharing BeH4 tetrahedra (there are 12BeH2
molecules in the unit cell) in contrast to the flat,
hydrogen-bridged, infinite chains previously thought
to exist in crystalline BeH2. Studies of the amorphous
form also find that it consists of a network of cornershared
tetrahedra. The density is 0.755 g/cm3.
Chemische Eigenschaften
Beryllium Hydride, BeH2 is an amorphous, colorless, highly toxic polymeric solid (H = 18.3%) that is stable to water but hydrolyzed by acid. It is insoluble in organic solvents but reacts with tertiary amines at 160 °C to form stable adducts, eg, (R3N· BeH2)2. Beryllium hydride was formerly of interest as a rocket fuel and as a moderator for nuclear reactors. Toxicity has been a serious barrier to its commercialization.
Physikalische Eigenschaften
White amorphous solid; density 0.65 g/cm
3; decomposes at 250°C; reacts with water.
Verwenden
Beryllium hydride (BeH2) liberates hydrogen gas when mixed with water. It is used as a source of hydrogen in experimental rockets and fuel cells. It has also been considered as a moderator for nuclear reactors. Methods of preparing and densifying the pure compound are described in a large number of patents. However, the toxicity has always been a serious barrier to its commercial exploitation.
synthetische
Beryllium hydride is made by treating an ethereal solution of beryllium borohydride with triphenylphosphine, or by pyrolysis of di-tert-butylberyllium.
Manufacturing Process
The only known method of
producing high purity beryllium hydride (95 –
98 wt % BeH2) is the pyrolysis of di-tert-butylberyllium diethyl etherate in a high boiling hydrocarbon at 200 ℃:
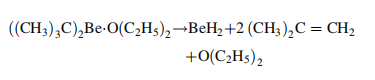
Sicherheitsprofil
Confirmed carcinogen. Adangerous fire hazard. When heated to 220°C it liberatesexplosive hydrogen gas. Reacts violently with methanol,water, and dilute acids. When heated to decomposition itemits toxic fumes of BeO. See BERYLLIUMCOMPOUNDS and HYDRIDE
Beryllium hydride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

