VIGABATRIN Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
Vigabatrin, the gamma-vinyl derivative of GABA, is a new anticonvulsant reportedly
effective in the treatment of intractable seizures unresponsive to currently available
therapy. Mechanistically vigabatrin is a potent irreversible GABA aminotransferase
inhibitor which modifies the enzyme's active-site by Michael addition. Other potential
indications have been suggested for vigabatrin, including depression and schizophrenia.
Chemische Eigenschaften
White or almost white powder.
Verwenden
Vigabatrin is a selective GABA transaminase inhibitor.
Definition
ChEBI: A gamma-amino acid having a gamma-vinyl GABA structure. It is an irreversible inhibitor of gamma-aminobutyric 664 acid transaminase
Weltgesundheitsorganisation (WHO)
Vigabatrin, an irreversible inhibitor of GABA-transaminase was
introduced in 1989 as a anticonvulsant for management of epilepsy unresponsive
to other antiepilepsy agents. In 1991 it was refused registration in Norway because
it induced toxic changes, including microvacuolation in the brain of two animal
species, at doses that are close to therapeutic dosage levels in man. It is still
marketed in Sweden and the United Kingdom.
Biologische Funktion
Vigabatrin (Sabril) is a relatively specific irreversible inhibitor
of GABA-transaminase (GABA-T), the major
enzyme responsible for the metabolism of GABA in the
mammalian CNS. As a result of inhibition of GABA-T,
there is an increase in the concentration of GABA in the
brain and consequently an increase in inhibitory neurotransmission.
Vigabatrin is well absorbed orally and is
distributed to all body systems.The major route of elimination
for vigabatrin is renal excretion of the parent compound;
no metabolites have been identified in humans.
At present, the primary indication for vigabatrin is
in the treatment of patients with partial seizures, but it
appears to be an effective and generally well tolerated
antiepileptic medication for other seizure types as well.
It should not be used in patients with absence epilepsy
or with myoclonic seizures. Vigabatrin is not approved
as an AED in the United States, although it is approved
in many other countries.
Allgemeine Beschreibung
Vigabatrin, a 4-vinyl analog of GABA, produces its pharmacologicalaction by irreversibly blocking GABA catabolismcatalyzed by GABA-T as discussed earlier. It is marketedin Europe and Canada as an adjunctive treatment ofpatients with partial seizures, but it has yet to gain FDA approvalin the United States even after extensive clinical trials.The main concern with this drug is its ability to causea reversible visual field defect associated with retinal functionin the eyes.
Biologische Aktivit?t
Selective GABA-T inhibitor. Anticonvulsant.
Synthese
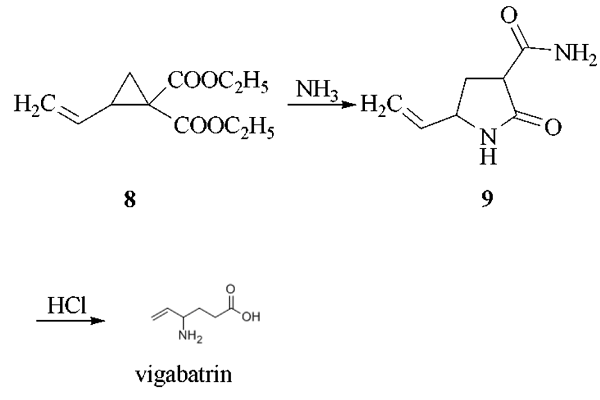
The reaction of 1,4-dichloro-2- butene with diethyl malonate in the presence of sodium ethoxide as catalyst in refluxing ethanol gives 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropane (8), which by reaction with gaseous ammonia in DMF is converted into 3-carboxamido- 5-vinyl-2-pyrrolidone (9). This compound is treated with HCl in refluxing acetic acid to yield vigabatrin.
VIGABATRIN Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

