5-Carboxyfluorescein Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
5-Carboxyfluorescein is a single isomer derivative of 5(6)-carboxyfluorescein that can be used to fluorescently label biomolecules through the interaction of carboxylic acid with primary amines. It demonstrates excitation/emission maxima of 492 and 518 nm, respectively.
Chemische Eigenschaften
Orange Solid
Verwenden
5-Carboxyfluorescein(5-FAM) contains a carboxylic acid that can be used to react with primary amines via carbodiimide activation of the carboxylic acid. It is a useful reagent for the preparation of hydrolytically stable fluorescent conjugates and is a useful starting material for the synthesis of other fluorescein-derives reagent. It has been principally used to develop a variety of green fluorescent reagents and small fluorescent molecules due to its relatively high absorbance characteristics, excellent fluorescence quantum yield, and good water solubility. The probe has also found application in studies of liposome-cell, cell-cell, and liposome-liposome interactions and in related studies on lipid bilayer structures.
Definition
ChEBI: 5-carboxyfluorescein is a monocarboxylic acid. It has a role as a fluorochrome. It is functionally related to a fluorescein (lactone form).
Biochem/physiol Actions
5-Carboxyfluorescein is used for the staining of cells to differentiate between live and dead cells. It helps in monitoring the integrity and intactness of the cell membrane. It is also used for the measurement of intracellular pH. 5-Carboxyfluorescein diacetate is a non-fluorescent molecule which is cleaved by intracellular esterases, thereby giving a green fluorescent product 5-carboxyfluorescein. In viable cells, the fluorescent molecule is impermeable to the cellular membrane.
Synthese
5-Carboxyfluorescein is a mixture of 5- and 6-isomers prepared by the reaction of benzotricarboxylic anhydride with resorcinol and zinc chloride.
Reaction: 1,2,4-Benzenetricarboxylic anhydride (also called 4-carboxyphthalic anhydride, 25.0 g, 0.13 mol) was added to a solution of 1,3-dihydroxybenzene (also called resorcinol, 28.6 g, 0.26 mol) in methane sulfonic acid (1M). An air condenser was attached to the flask and the reaction was heated at 85°C in an open vessel for 24 h. After cooling to room temperature, the reaction mixture was poured into 7 volumes of ice/water. An orange-yellow precipitate formed; this was collected by filtration and dried in an oven at 200°C. This residue was recrystallized two times from methanol/hexane to give 1.0 g of 6-carboxyfluorescein methanesulfonic acid adduct 2b. The mother liquors from this procedure were collected, the solvent was removed in vacuo, and the residues were recrystallized two times from ethanol/hexanes to give 3.2 g of 5-carboxyfluorescein methanesulfonic acid adduct 2a.
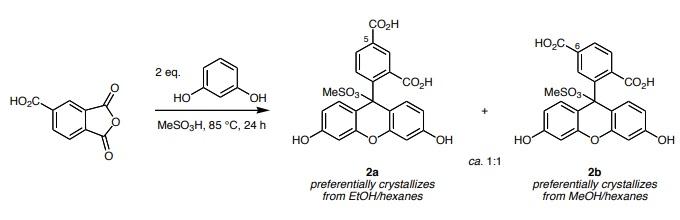
The methane sulfonic esters 2 are easily converted to the 5- and 6-carboxyfluoresceins 1 by treatment with sodium hydroxide solution then neutralizing with aqueous HCl.
5-Carboxyfluorescein Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

