6-Dimethylaminopurin Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R25:Giftig beim Verschlucken.
R27:Sehr giftig bei Berührung mit der Haut.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S28:Bei Berührung mit der Haut sofort abwaschen mit viel . . . (vom Hersteller anzugeben).
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S24/25:Berührung mit den Augen und der Haut vermeiden.
S22:Staub nicht einatmen.
Chemische Eigenschaften
white to light yellow crystal powder
Verwenden
6-Dimethylaminopurine is a serine threonine protein kinase inhibitor. It inhibits the germinal vesicle breakdown and the meiotic maturation of oocytes. It can be used to rtificially lengthen the pre-maturation period of oocyte growth, in vitro, by inhibiting germinal vesicle breakdown in mouse and human oocytes.
Application
6-(Dimethylamino)purine has been used:
as a supplement in GR-1 aa medium (bovine medium) for parthenogenetic activation of bovine oocytes to study its potential for embryo development.
in the activation step during the production of nuclear transfer embryos.
as a supplement in HCR2aa medium to activate interspecies embryos derived from interspecies somatic cell nuclear transfer (iSCNT) technique.
A purine antagonist.
In the benzodiazepine receptor (BZR) binding assay, it inhibits the binding of 1.5 nM [3H]diazepam at 100uM in rat brains.
synthetische
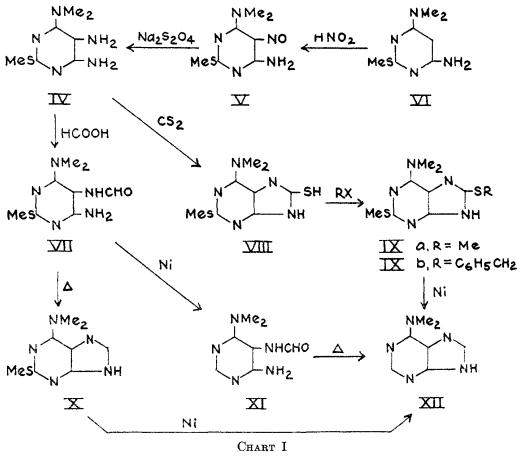
6-Dimethylaminopurine synthesis: 2-Methylmercapto-4-amino-6-dimethylaminopyrimidine (VI) was smoothly nitrosated in 10% acetic acid to the 5-nitrosopyrimidine (V) in 95% yield. Reduction of V with sodium hydrosulfite to the triamine (IV), followed by formylation gave the 5-formamidopyrimidine (VII) in 76% over-all yield for the two steps. Reductive formylation of V directly to VI1 with zinc and formic acid, although more rapid, was less efficient (50% yield). Ring closure of VII to 2-methyhercapto-6-dimethylaminopurine (X) was best done on a small scale by short fusion at 250°(99% yield), although boiling quinoline, formamide, or dilute alcoholic sodium hydroxide could also be employed. The latter reagent was most efficient on a large scale. Desulfurization of X with Raney nickel (7) in 1 N sodium hydroxide at 100° afforded the final product, 6-dimethylaminopurine (XII) in 43% yield.This compound was identical in all respects with the C7H9N5 moiety from puromycin (2).
Definition
ChEBI: 6-Dimethylaminopurine is a tertiary amine that is adenine substituted at N-6 by geminal methyl groups. It is functionally related to an adenine.
Origin
6-Dimethylaminopurine is a puromycin analog that was first identified in the spores of Streptomyces alboniger (PMID: 5019066 ). It has subsequently been identified in several algae species (PMID: 4206669 ).
Allgemeine Beschreibung
6-(Dimethylamino)purine (6-DMAP) is a purine-based metabolite with two condensed heterocyclic rings and two methyl groups linked to the amino group of the purine unit of adenine.
Biochem/physiol Actions
6-(Dimethylamino)purine (6-DMAP) is a protein kinase and cyclin-dependent kinase inhibitor. It acts as a secondary metabolite and mediates RNA modification. 6-DMAP is a potent cytokinetic inhibitor and is used in parthenogenesis and meiosis studies. It is also used to promote pronuclei formation in mammalian oocytes. 6-DMAP is a dual fluorescence molecule according to femtosecond fluorescence up-conversion spectroscopy studies.
l?uterung methode
It is purified by recrystallisation from H2O, EtOH (0.32g in 10mL) or CHCl3. [Albert & Brown J Chem Soc 2060 1954, UV: Mason J Chem Soc 2071 1954.] The monohydrochloride crystallises from EtOH/Et2O, m 2 5 3o(dec) [Elion et al. J Am Chem Soc 74 411 1952], the dihydrochloride has m 225o(dec) and the picrate has m 245o (235-236.5o) [Fryth et al. J Am Chem Soc 80 2736 1958]. [Beilstein 26 III/IV 3566.]
6-Dimethylaminopurin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

