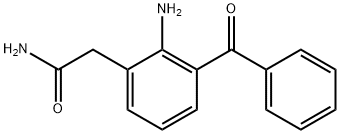
Nepafenac
|
|
Nepafenac Eigenschaften
- Schmelzpunkt:
- 177-181°C
- Siedepunkt:
- 562.5±50.0 °C(Predicted)
- Dichte
- 1.249±0.06 g/cm3(Predicted)
- storage temp.
- room temp
- L?slichkeit
- DMSO: ≥5mg/mL
- pka
- 16.09±0.40(Predicted)
- Aggregatzustand
- powder
- Farbe
- faint yellow to dark yellow
- Wasserl?slichkeit
- 0.014 mg/mL in water
- Merck
- 14,6469
- InChI
- InChI=1S/C15H14N2O2/c16-13(18)9-11-7-4-8-12(14(11)17)15(19)10-5-2-1-3-6-10/h1-8H,9,17H2,(H2,16,18)
- InChIKey
- QEFAQIPZVLVERP-UHFFFAOYSA-N
- SMILES
- C1(CC(N)=O)=CC=CC(C(=O)C2=CC=CC=C2)=C1N
- CAS Datenbank
- 78281-72-8(CAS DataBase Reference)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | N | ||
|---|---|---|---|
| R-S?tze: | 50/53 | ||
| S-S?tze: | 60-61 | ||
| RIDADR | UN 3077 9 / PGIII | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | CY1480710 | ||
| HS Code | 2924.29.6250 |
| Bildanzeige (GHS) |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Nepafenac Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Nepafenac, launched by Alcon Laboratories, is a topical ophthalmic medication indicated for the treatment of ocular pain and inflammation associated with cataract surgery. Nepafenac is a prodrug of amfenac, which is an NSAID and a potent non-selective inhibitor of COX-1 (IC50=0.25 μM)) and COX-2 (IC50=0.15μM). Nepefenac itself exhibits only weak activity against COX-1 (IC50=64.3μM). Amfenac (Fenazox) has been marketed in Japan since 1986 for the treatment of rheumatoid arthritis, post-surgical pain, and inflammation. With most NSAIDs that are currently being used as topical ophthalmic agents, the maximum drug concentration is achieved on the ocular surface, with progressively lower concentrations in the cornea, aqueous humor, vitreous, and retina. Nepafenac has been found to have a penetration coefficient that is 4-28 times greater than that achieved with conventional NSAIDs such as diclofenac, bromofenac, and ketorolac. In addition, the bioconversion of nepefenac to amfenac is primarily mediated by ocular tissue hydrolases, specifically in the iris, ciliary body, retina, and choroid. The enhanced permeability of nepefenac combined with rapid bioactivation in the ocular tissue translates into superior anti-inflammatory efficacy at the target sites.Chemische Eigenschaften
Nepafenac is a light Yellow Solid or powder. It is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF).Verwenden
Nepafenac is a non-steroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop. It reduces pain and inflammation in the eyes. Nepafenac ophthalmic is used to reduce pain and swelling after cataract surgery.Definition
ChEBI: Nepafenac is a monocarboxylic acid amide that is amfenac in which the carboxylic acid group has been converted into the corresponding carboxamide. It is a prodrug for amfenac, used in eye drops to treat pain and inflammation following cataract surgery. It has a role as a prodrug, a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a non-steroidal anti-inflammatory drug and a non-narcotic analgesic.Clinical Use
Nepafenac is a novel ophthalmic non-steroidal anti-inflammatory drug (NSAID), for the treatment of eye pain and inflammation caused by cataract surgery, compared with traditional NSAIDs, chemical structure of Nepafenac is conducive to make it rapidly penetrate the cornea and distribute to its target site, which is helpful to reduce the accumulation of the drug in the corneal surface and to reduce the incidence of complications of the eye surface, it has many advantages such as infiltration, targeting strong, little toxic side effects and so on.August 19, 2005 ,the US Food and Drug Administration (FDA) approved nepafenac ophthalmic suspension for the treatment of cataract surgery-related pain and inflammation, it is the first ophthalmic NSAID prodrug formulation approved for marketing.
Nepafenac after ocular administration, can rapidly pass through the cornea , and under the action of eye tissue hydrolytic enzymes,it can become into ammonia diclofenac (a kind of NSAID); and ammonia diclofenac by inhibiting prostaglandin H synthase ( cyclooxygenase), can block prostaglandin synthesis to play its role as an anti-inflammatory analgesic. As is known, prostaglandin is one of the media causing ocular inflammation it can lead to blood-aqueous barrier crash, vasodilatation, increased vascular permeability and leukocyte chemotaxis, etc. In addition, prostaglandins can also control contraction of the iris sphincter through non-cholinergic mechanism which can trigger the miosis reaction during eye surgery and after surgery. After ocular administration of NSAIDs, it can inhibit prostaglandins synthesis in the iris, ciliary body and conjunctiva, so people can prevent eye inflammation, and reduce the associated pain.
The above information is edited by the chemicalbook of Tian Ye.
Mode of action
Nepafenac is a non-steroidal anti-inflammatory and analgesic prodrug. After topical ocular dosing, nepafenac penetrates the cornea and is converted by ocular tissue hydrolases to amfenac, a nonsteroidal anti-inflammatory drug. Amfenac inhibits the action of prostaglandin H synthase (cyclooxygenase), an enzyme required for prostaglandin production.Nepafenac Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Nepafenac Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 382)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Shandong Hengshannuode Pharmaceutical Technology Co., Ltd. | +8615065888978 |
admin@hsnordpharma.com | China | 92 | 58 |
| Tianjin Kilo Pharmaceutical Sci-Tech Co., Ltd | +86-02223869539 +86-15560057295 |
trade.kilopharma@foxmail.com | China | 27 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 987 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-18712993135 |
sales01@tairunfaz.com | China | 8051 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 |
niki@zlchemi.com | China | 7245 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 3237 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3009 | 60 |
78281-72-8()Verwandte Suche:
4-Aminobenzoesure
2-Aminoacetamid
N-Acetylsulfanilylchlorid
Glycin
2-Phenylacetamid
Harnstoff
Betain
Acetamid
ALTRENOGEST
Anthranilic acid
AMINO ACIDS
- AHR 9434
- AL 6515
- Nepafenac
- 2-(2-amino-3-benzoyl-phenyl)acetamide
- 2-Amino-3-benzoylbenzeneacetamide
- Nepafenac - WS
- nepafenac,Nevance
- Nepafanac
- (R)-(but-3-yn-2-yloxy)trimethylsilane
- Nepafenac, 99%, a selective COX-2 inhibitor
- CS-328
- Nepafenac>
- Benzeneacetamide, 2-amino-3-benzoyl-
- Nepafenac USP/EP/BP
- Nepafenac API
- Nepafenac (AHR9434
- NepafenacQ: What is Nepafenac Q: What is the CAS Number of Nepafenac Q: What is the storage condition of Nepafenac Q: What are the applications of Nepafenac
- Nevanac
- Nepafenac/AHR9434/AL6515/Nevanac
- NEPAFENAC STD
- Nepafenac (200 mg)
- Nepafenac, 10 mM in DMSO
- 78281-72-8
- 8281-72-8
- C15H9D5N2O2
- Nevanac
- Other APIs
- Amines
- Aromatics
- Intermediates & Fine Chemicals
- Isotope Labelled Compounds
- Pharmaceutical intermediate
- Pharmaceuticals
- API