| Identification | More | [Name]
4-Chloro-3-nitrobenzenesulfonamide | [CAS]
97-09-6 | [Synonyms]
3-NITRO-4-CHLOROBENZENESULFONAMIDE
4-CHLORO-3-NITROBENZENE-1-SULFONAMIDE
4-CHLORO-3-NITROBENZENESULFONAMIDE
4-CHLORO-3-NITROSULFAMYLBENZENE
BUTTPARK 76\07-88
O-NITROCHLOROBENZENE-P-SULFONAMIDE
YELLOW SULFONE
4-chloro-3-nitro-benzenesulfonamid
4-chloro-3-nitrobenzenesulphonamide
4-Chloro-3-nitrobenzene-1-sulphonamide
3-Nitro-4-chlorobenzensulfonyl amine
2-NITROCHLOROBENZENE-4-SULFONAMIDE | [EINECS(EC#)]
202-559-8 | [Molecular Formula]
C6H5ClN2O4S | [MDL Number]
MFCD00035783 | [Molecular Weight]
236.63 | [MOL File]
97-09-6.mol |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow to light brown crystalline powder | [Uses]
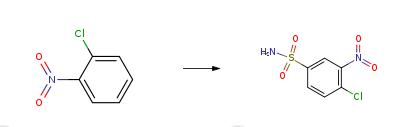
4-Chloro-3-nitrobenzenesulfonamide is used as a chemical intermediate for azo dyes. | [Synthesis]
Chlorosulphonic acid (450 mL) was slowly added to 2-chloronitrobenzene (100 g). The reaction mass was heated to 100°C and maintained at that temperat ure for 6 hours before cooling to ambient temperature and stirring for an additional 12 hours. The reaction mass was slowly poured into chilled aqueous ammonia (800 mL) and the reaction mixture was stirred for 3 hours at -10°C. The reaction mixture was then warmed to 23°C and stirred for 2 h ours. The reaction mixture was then filtered and the obtained solid was washed three times with water (200 mL x 3). The solid was dissolved in methanol (600 mL) at 60°C, charged with water (200 mL), and was stirred for 1 hour at 60°C. Again, the reaction mixture was charged with water (200 mL) and stirred for 1 hour at 60°C. Once again, the reaction mixtu re was charged with water (200 mL) and stirred for 1 hour. After cooling to room temperature and stirring for 1 hour, the reaction mixture was filtered, and the solid was washed with a 1 :1 mixture of methanol and water (100 mL). The solid was dried under vacuum at 60°C. Crystallization of the residue with toluen e gave the 4-Chloro-3-nitrobenzenesulfonamide(50 g).
 |
|
|