| Identification | More | [Name]
PILOCARPINE | [CAS]
92-13-7 | [Synonyms]
PILOCARPINE
(+)-pilocarpin
(3S-cis)-3-Ethyldihydro-4-((1-methyl-1H-imidazol-5-yl)methyl)-2(3H)-furanone
(3s-cis)-3-ethyldihydro-4-[(1-methyl-1h-imidazol-5-yl)methyl]-2(3h)-furanone
3-Ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]dihydro-2(3H)-furanone
3-ethyldihydro-4-((1-methyl-1h-imidazol-5-yl)methyl)-2(3h)-furanon(3s-ci
3-Ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-2(3H)-furanone
actone
alpha-ethyl-beta-(hydroxymethyl)-1-methyl-imidazole-5-butyricacigamma-l
alpha-ethyl-beta-(hydroxymethyl)-1-methyl-imidazole-5-butyricacigamma-lac
Imidazole-5-butyric acid, alpha-ethyl-beta-(hydroxymethyl)-1-methyl-, gamma-lactone
Ocusert P 20
Ocusert Pilo
ocusertp20
ocusertpilo
Pilocarpin
Pilocarpol
Pilokarpin
pilokarpol
Syncarpine | [EINECS(EC#)]
202-128-4 | [Molecular Formula]
C11H16N2O2 | [MDL Number]
MFCD00153042 | [Molecular Weight]
208.26 | [MOL File]
92-13-7.mol |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R26/28:Very Toxic by inhalation and if swallowed . | [Safety Statements ]
S25:Avoid contact with eyes .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
1544 | [WGK Germany ]
3 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2939800000 | [Safety Profile]
A human poison by
subcutaneous route. Poison experimentally
by ingestion, intravenous, intraperitoneal,
and subcutaneous routes. A very poisonous
alkaloid that is used to remove excess fluid
accumulations from the body. Its action on
the sweat glands makes it a powerful
sudorific. It very rarely causes death, but,
when it does, it is by paralysis of the heart or
edema of the lungs. Dangerous; on heating
to decomposition it emits toxic fumes of
NOx. | [Hazardous Substances Data]
92-13-7(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
Pilocarpine acts by stimulating muscarinic receptors, therefore making it similar in action
to acetylcholine when systematically introduced. This compound differs from acetyl�choline in that it does not react with any nicotinic receptors, but by stimulating the CNS.
Its effects are blocked by atropine. It has found therapeutic use in ophthalmology as a
myotic agent. | [Chemical Properties]
Colorless crystalline solid or an oil; melts at34°C (93.2°F); dissolves in water, alcohol,and chloroform; slightly soluble in ether andbenzene. | [Physical properties]
Appearance: colorless crystal or white crystalline powder. Solubility: freely soluble
in water; slightly soluble in ethanol; insoluble in chloroform or diethyl ether.
Melting point: 174–178?°C. | [Originator]
Andre Carpine, Andre Laboratries Pvt. Ltd., India | [History]
It has a history of hundreds of years since pilocarpine was used to treat glaucoma
.
In 1933, the chemical synthesis of pilocarpine was firstly reported. However,
pilocarpine couldn’t be used for treatments, because its synthetic route is so long
and focuses on isopilocarpine, of which the pharmacological activities are 1/20–
1/50 of pilocarpine. In 1972, DeGraw successfully synthesized the cis-homopilopic
acid employing catalytic hydrogenation of precious metals and obtained pilocarpine
as the main part product. Therefore, the study on the production of pilocarpine by
chemical synthesis has made new progress and has been artificially synthesized . | [Uses]
Cholinergic (ophthalmic). | [Uses]
Pilocarpine occurs in the leaves of variousspecies of pilocarpus. It is used as an antidotefor atropine poisoning and in ophthalmologyto produce contraction of the pupil. | [Definition]
ChEBI: The (+)-enantiomer of pilocarpine. | [Manufacturing Process]
The 1-methylimidazole-5-aldehyde is easily accessible from sarcosine methyl ester hydrochloride and dimethylamino-2-azaprop-2-en-1
Pilocarpine ylidenedimethylammonium.
0.14 mol of ethyl diethylphosphonoethoxyacetate is slowly added dropwise with stirring and under inert gas to a suspension of 0.14 mol of NaH (paraffinfree) in 250 ml of abs. THF, the mixture is stirred for 1 h at 20°C and a solution of 0.093 mol of 1-methylimidazole-5-aldehyde in 100 ml of abs. THF is added dropwise. After stirring at 20°C for 10 min, the solvent is distilled off in vacuo, the residue is taken up in a little H2O, and the solution is acidified with 1 N HCl and washed several times with ether. The aqueous phase is rendered alkaline using 2 N NaOH with cooling (0°-5°C) and extracted several times with CH2Cl2. After drying of the organic extracts with Na2SO4, the solvent is removed in vacuo and 2-ethoxy-3-[(1-methyl-1H-imidazol-5yl)methyl]-acrilic acid ethyl ester. Yield: 99% of theory.
122 ml of 45% diisobutylaluminum hydride solution (328 mmol) are slowly added dropwise under inert gas, with stirring and ice cooling, to a solution of 137 mmol of 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-acrilic acid ethyl ester in 600 ml of abs. C6H6. Stirring of the mixture is continued for a further 30 min at 0°-5°C and 600 ml of CH3OH, then 100 ml of H2O, are slowly added. The hydroxide precipitate is filtered off with suction and washed several times with hot CH3OH. After drying of the combined filtrates the solvents are distilled off in vacuo and the residue is crystallized using C2H5OH. 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-prop-2-en-1-ol was obtained. Yield: 100% of theory. The crude product is pure enough for the subsequent reaction. Recrystallization of an analytical sample from CH3OH/acetone: melting point 129°C.
A solution of 58 mmol of 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]prop-2-en-1-ol in 116.6 ml of HCl (= 116.6 mmol) is stirred at 30°-35°C for 1.5 h and concentrated in vacuo at the same temperature. The residual HCl is removed by distillation with CHCl3 in vacuo. After seeding, the residue crystallizes at 20°C (15 h) 1-hydroxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]propan-2-one hydrochloride. The crystallizate is filtered off with suction, washed with a little CH3OH and dried in vacuo. Yield: 86% of theory; melting point 190°C.
About 80-90% of the equivalent amount of NaOCH3 solution in CH3OH is slowly added dropwise at 20°C with stirring and exclusion of moisture to a suspension of 21.24 mmol of 1-hydroxy-3-[(1-methyl-1H-imidazol-5yl)methyl]-propan-2-one hydrochloride in 80 ml of CH3OH, in the course of which the pH of 6.5 is not to be exceeded. The solvent is distilled off in vacuo at a maximum of 30°C and the residue of 1-hydroxy-3-[(1-methyl-1Himidazol-5-yl)methyl]-propan-2-one is purified by flash chromatography (silica gel; CHCl3/CH3OH). Yield: 100% of theory; viscous, orange-colored oil.
Catalytic amounts of 4-dimethylaminopyridine and a solution of 21.3 mmol of 1-hydroxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-propan-2-one in 80 ml of CH2Cl2 are added to a solution of 26.44 mmol of 2-diethylphosphonobutyric acid in 40 ml of purified CH2Cl2. After cooling to 0°-5°C, a solution of 23.5 mmol of dicyclohexylcarbodiimide in 60 ml of CH2Cl2 is added dropwise and the mixture is stirred for 1 h at 0°-5°C and for 2 h at 20°C. The crystallizeddicyclohexylurea is filtered off with suction and the filtrate is washed with H2O and saturated NaHCO3 solution. After drying of the organic phase the solvent is distilled off at 30°C in vacuo and the residue of 2-diethoxy-phosphoryl)butyric acid 3-[(1-methyl-1H-imidazol-5-yl)methyl]-2-oxo-propyl ester is purified by flash chromatography (silica gel; ethyl acetate/CH3OH). Yield: 95% of theory of a viscous, orange-colored oil.
A mixture of 5 mmol each of 80% NaH and 15-crown-5 in 50 ml of absol. toluene is stirred at 20°C under inert gas for 10 min and a solution of 5 mmol of 2-diethoxy-phosphoryl)-butiric acid 3-[(1-methyl-1H-imidazol-5-yl)methyl]2-oxo-propyl ester in 50 ml of absol. toluene is then added dropwise. Stirring is continued for a further 15 min under inert gas and the mixture is hydrolyzed with a little water until phase separation is detectable. After separating off the organic phase, the aqueous layer is saturated with NaCl and extracted several times with CHCl3. The combined organic phases are the solvent is distilled off at 40°C in vacuo and the residue 3-ethyl-4-[(1-methyl1H-imidazol-5-yl)methyl]-5H-furan-2-one is purified twice by flash chromatography (silica gel; ethyl acetate/CH3OH). Yield: 52% of theory; virtually colorless oil.
1.36 mmol of 3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]-5H-furan-2-one in 15.5 ml of CH3OH are hydrogenated for 5 h at 50 bar and 60°C using 210 mg of Pd/carbon (10%). After filtering off the catalyst and distilling off the solvent at 30°C in vacuo, the oily residue (about 250 mg) is treated with 10 ml of 1 N HCl and the mixture is stirred for 3 h at 20°C. The hydrochloric acid is distilled off in vacuo at 35°-40°C, the oily residue is taken up in a little CH3OH and ether is added. The precipitate of pilocarpine hydrochloride is recrystallized from CH3OH/ether. Yield: 73% of theory; melting point 210°C. | [Brand name]
Pilopine (Alcon);
Salagen (Millot Laboratories, France). | [Therapeutic Function]
Cholinergic | [Health Hazard]
Pilocarpine is a tropane alkaloid. Toxicsymptoms are characterized by muscariniceffects. Toxic effects include hypersecretionof saliva, sweat, and tears; contraction of thepupils of the eyes; and gastric pain accom panied with nausea, vomiting, and diarrhea.Other symptoms are excitability, twitching,and lowering of blood pressure. High dosesmay lead to death due to respiratory failure.A lethal dose in humans is estimated withinthe range of 150–200 mg. | [Pharmacology]
Pilocarpine activates cholinergic M-receptor and has an obvious effect on eyes and
salivary glands. Pilocarpine nitrate eye drops take part in the actions of myosis,
depressing intraocular pressure and alleviating cyclospasm. It increases glandular
secretions at 10–20? mg, i.h., including the sweat gland, salivary gland, lacrimal
gland, gastric gland, pancreas, intestinal gland, respiratory mucosa, and so on.
Pilocarpine activates intestinal smooth muscle and promotes its tension and peristalsis. It induces asthma by activating bronchial smooth muscle and activates
smooth muscles of uterus, bladder, gallbladder, and biliary passage as well | [Clinical Use]
Pilocarpine nitrate is mainly used to treat glaucoma clinically. Characterized with
the progressive cupping of the optic disk, hypopsia, and elevated intraocular pressure, the severe patients will go blind. Patients with angle-closure glaucoma (congestive glaucoma) generally have the narrow anterior chamber angle, the obstruction
of aqueous humor outflow, and the elevation of intraocular pressure, and these can
be reversed by a low-concentration pilocarpine. But it is noted that a highconcentration pilocarpine will promote the progress of glaucoma. Pilocarpine is
also used to treat open-angle glaucoma. The mechanism of the action is not entirely
clear. Using atropine and pilocarpine alternately prevents posterior synechiae. In
addition, pilocarpine is orally used to treat Zagari’s disease after neck radiotherapy,
increasing salivary secretion and sweat secretion | [Synthesis]
Pilocarpine, 3-ethyl-4-(1-methyl-5-imidazolymethyl)tetrahydrofuran-2-one
(13.1.22), is an alkaloid that is made from leaves of the tropic plant Pilocarpus jaborandi.
It is synthesized in a few different ways [25¨C32], the most relevant of which seems to be
from 2-ethyl-3-carboxy-2-butyrolactone [25¨C27], which with the help of thionyl chloride
is turned into the acid chloride (13.1.15) and further reacted with diazomethane and
ethanol, to give the corresponding ethyl ester (Arndt¨CEistert reaction), which is hydrolyzed
into the acid (13.1.16). The resulting acid (13.1.16) is again changed into the acid chloride
(13.1.17) by thionyl chloride. The obtained acid chloride is treated with diazomethane. But
in this case the intermediate forming ketene is treated with hydrogen chloride to give the
chloroketone (13.1.18). Reacting this with potassium phthalimide and subsequent removal
of the phthalimide protecting group by acid hydrolysis gives the aminoketone (13.1.19),
which is reacted with an acidic solution of potassium thiocyanate, forming 3-ethyl-4-(2-
mercapto-5-imidazolylmethyl)tetrahydrofuran-2-one (13.1.20). Mild oxidation of this
product allows to remove the mercapto- group from the product (13.1.20), giving 3-ethyl-
4-(5-imidazolylmethyl)tetrahydrofuran-2-one (13.1.21). Alkylation of the resulting prod�uct with methyl iodide leads to the formation of pilocarpine (13.1.22).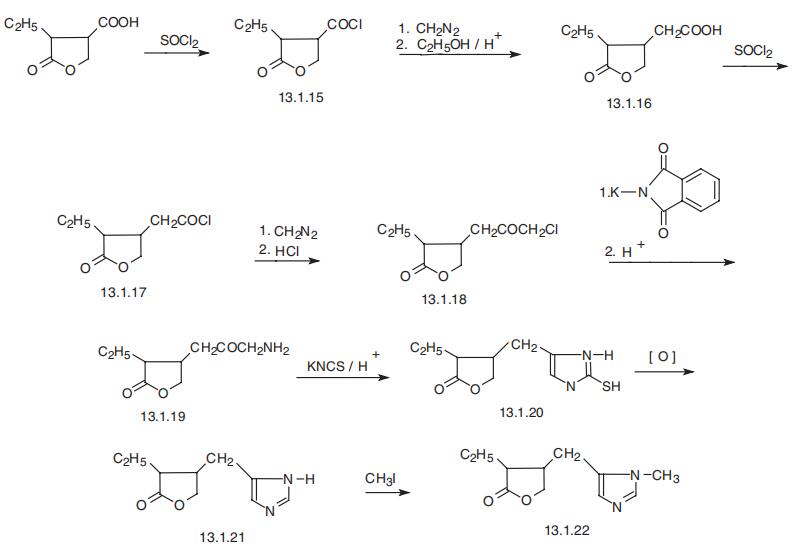
| [References]
Hardy., Bull. Soc. Chim. Fr., 24,497 (1875)
Gerrard., Pharm. J., 5,865,965 (1875)
Gerrard., ibid, 7,255 (1877)
Hardy, Calmels., Compt. rend., 102,1116,1251,1562 (1886)
Wagenaar., Pharm. Weekbl., 67, 285 (1930)
Preobrashenski et al., Ber., 63,460 (1930)
Preobrashenskietal., ibid, 69, 1835 (1936)
Roche, Lynch., Analyst, 73,311 (1948)
Pharmacology:
Hollander., Gastroenterology, 2, 20 I (1944) |
|
|