| Identification | More | [Name]
Lithium bis(trifluoromethanesulphonyl)imide | [CAS]
90076-65-6 | [Synonyms]
BISTRIFLUOROMETHANESULFONIMIDE LITHIUM SALT
BIS(TRIFLUOROMETHANESULFONYL)IMIDE LITHIUM SALT
BIS(TRIFLUOROMETHYLSULFONYL)AMINE LITHIUM SALT
LITHIUM BISTRIFLUOROMETHANESULFONIMIDATE
LITHIUM BIS(TRIFLUOROMETHANESULFONIMIDE)
LITHIUM BIS(TRIFLUOROMETHANESULFONYL)IMIDE
LITHIUM BIS(TRIFLUOROMETHANESULPHONYL)IMIDE
LITHIUM TRIFLUOROMETHANESULFONIMIDE
N-LITHIOTRIFLUOROMETHANESULFONIMIDE
1,1,1-trifluoro-n-[(trifluoromethyl)sulfonyl]-methanesulfonamidlithiumsalt
lithiumbis(trifluoromethylsulfonyl)imide
Methanesulfonamide,1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]-,lithiumsalt
bis(Trifluoromethanesulphonyl)imide, lithium salt
Bis(trifluoromethane)sulfonimide lithium salt, 99.95% metals basis
Lithium bis(trifluoromethanesulphonyl)imide 99%
Lithiumbis(trifluoromethanesulphonyl)imide99%
1,1,1-Trifluoro-N-[(trifluoromethyl)sulfonyl]methanesulfon amide lithium salt
Lithium bis(trifluoromethylsulfonyl)imide, 98+%
Bis(trifluoromethylsulfonyl)amine lithium salt, Lithium bistrifluoromethanesulfonimidate | [EINECS(EC#)]
415-300-0 | [Molecular Formula]
C2F6LiNO4S2 | [MDL Number]
MFCD00210017 | [Molecular Weight]
287.09 | [MOL File]
90076-65-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White hygroscopic powder | [Melting point ]
234-238 °C(lit.)
| [density ]
1,334 g/cm3 | [vapor pressure ]
0Pa at 25℃ | [Fp ]
>100°C (>212°F) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
H2O: 10 mg/mL, clear, colorless
| [form ]
Hygroscopic Powder | [color ]
White | [Specific Gravity]
1.334 | [Water Solubility ]
Soluble in water. | [Sensitive ]
Moisture Sensitive | [BRN ]
6625414 | [InChIKey]
QSZMZKBZAYQGRS-UHFFFAOYSA-N | [LogP]
-1.46 | [CAS DataBase Reference]
90076-65-6(CAS DataBase Reference) | [EPA Substance Registry System]
90076-65-6(EPA Substance) | [Boiling point ]
234-238?°C (lit.) | [Description]
Lithium bis(trifluoromethylsulphonyl)imide (LiTFSI) is normally used as a p-dopant?to enhance the conductivity and hole mobility of the?Spiro-OMeTAD for perovskite solar cells. It is believed that The function of LiTFSI in PSCs is?similar to that in solid-state dye-sensitised solar cells [2]. |
| Safety Data | Back Directory | [Hazard Codes ]
T,C | [Risk Statements ]
R24/25:Toxic in contact with skin and if swallowed .
R34:Causes burns.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 2923 8/PG 2
| [WGK Germany ]
2
| [Hazard Note ]
Harmful/Corrosive/Moisture Sensitive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29309090 |
| Hazard Information | Back Directory | [Chemical Properties]
White hygroscopic powder | [Uses]
An electrolyte | [Uses]
It finds application in the preparation of rare-earth Lewis acid catalysts. | [General Description]
Bis(trifluoromethane)sulfonimide lithium salt is a synthetic reagent. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
5.91 g of anhydrous lithium fluoride and N-butyl-bistrifluoromethylsulfonimide 51.26 g. Add to 250 g of isopropyl acetate and reflux for 10 hours. It was filtered after being cooled to room temperature, and the filtrate was concentrated to dryness under reduced pressure.200 g of dichloromethane was added dropwise, and the mixture was stirred at 20 ° C for 2 hours and then filtered. Wash with dichloromethane. The filter cake was dried at 80 ° C to obtain 39.57 g of Lithium bis(trifluoromethanesulphonyl)imide. The yield is 90.7%.
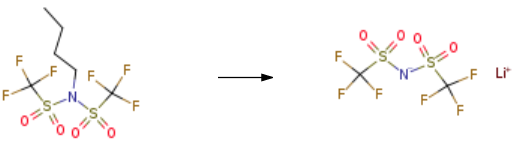
| [Properties and Applications]
Lithium bis(trifluoromethanesulphonyl)imide (LiTFSI) is a hydrophilic organic salt with many uses in electric and electronic systems. Its bis(trifluoromethane)sulfonimide anion, often called bistriflimide, is helpful in coordinating weakly with cations. LiTFSI's other important property is its extremely high solubility in water: 21 molal or ≈6 kg/L of solution. LiTFSI is safer than the formerly used salt, lithium hexafluorophosphate (LiPF6). To improve the cells' ability to transport electrical charges, researchers are doped with a combination of LiTFSI and a semiconductor called Spiro-OMeTAD1; however, this process is extremely slow. Taylor and his fellow researchers solved the problem by bubbling carbon dioxide into a solution of spiro-OMeTAD and LiTFSI while irradiating the mixture with ultraviolet light. They then cast a film from the solution onto the perovskite light absorber. The process can be completed in ≈1 minute, compared with the older, hours-long doping procedure. | [Toxics Screening Level]
There are two Initial Threshold Screening Levels (ITSLs) for lithium bis(trifluoromethane sulfonyl)imide (LiTFMSI) (CAS No. 90076-65-6):
Acute ITSL: 40 μg/m3 with a 24-hr. averaging time, and Chronic ITSL: 1 μg/m3 with an annual averaging time |
|
|