| Identification | More | [Name]
Ofloxacin | [CAS]
82419-36-1 | [Synonyms]
9,10-Difluoro-2,3-dihydro-3-methyl-7-oxo-(3S)-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid
(+/-)-9-fluoro-2, 3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7h-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid
AKOS NCG1-0050
floxin
LEVOFLOXACIN
LEVOFLOXACIN CARBOXYLIC ACID
l-ofloxacin
lvfx
OFLOXACIN
oflx
ophthalmic
rwj 25213
(-)-(s)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazin-yl)-7-oxo-7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid
TARIVID
(+-)-ethyl-10-(4-methyl-1-piperazinyl)-7-oxo
dl-8280
hoe280
oflocet
ofloxacine
orf18489 | [EINECS(EC#)]
680-263-1 | [Molecular Formula]
C18H20FN3O4 | [MDL Number]
MFCD00226105 | [Molecular Weight]
361.37 | [MOL File]
82419-36-1.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Solid | [Melting point ]
270-2750C | [Boiling point ]
571.5±50.0 °C(Predicted) | [density ]
1.2688 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
1 M NaOH: soluble50mg/mL | [form ]
neat | [pka]
5.19±0.40(Predicted) | [color ]
Colorless needles from ethanol | [biological source]
synthetic | [Water Solubility ]
Soluble in acetic acid or water. Slightly soluble in methanol | [Usage]
Fluorinated quinolone antibacterial | [λmax]
326nm(H2O)(lit.) | [Merck ]
14,6771 | [BCS Class]
1 | [CAS DataBase Reference]
82419-36-1(CAS DataBase Reference) | [EPA Substance Registry System]
82419-36-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact .
R68:Possible risk of irreversible effects.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S24/25:Avoid contact with skin and eyes .
S22:Do not breathe dust .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
UU8815550
| [HS Code ]
29349900 | [Hazardous Substances Data]
82419-36-1(Hazardous Substances Data) | [Toxicity]
LD50 in male, female mice, male, female rats (mg/kg): 5450, 5290, 3590, 3750 orally; 208, 233, 273, 276 i.v.; >10000, >10000, 7070, 9000 s.c. (Ohno) |
| Hazard Information | Back Directory | [Description]
Ofloxacin is an antibacterial agent with increased potency in comparison to the
prototype third generation quinolone, norfloxacin. It has a broad spectrum of
activity against gram-positive and gram-negative bacteria and is useful in the
treatment of kidney, genitourinary, and upper respiratory tract infections. | [Chemical Properties]
Off-White Solid | [Originator]
Daiichi Seiyaku (Japan) | [Uses]
Fluorinated quinolone antibacterial | [Definition]
ChEBI: An oxazinoquinolone carrying carboxy, fluoro, methyl and 4-methylpiperazino substituents. A synthetic fluoroquinolone antibacterial agent, it inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication. | [Indications]
Ofloxacin also possesses a broad spectrum of antimicrobial action. It is highly active with
respect to Gram-negative microorganisms, such as blue-pus bacillus, hemophilic and colon
bacillus, shigella, salmonella, and chlamydia. It is used for infections of the respiratory
tract, ears, throat, nose, skin, soft tissue, bones, joints, infective-inflammatory diseases
of the abdominal cavity organs (kidneys, urinary tract), and organs of the pelvis minor
(genitalia), and for gonorrhea. Synonyms of this drug are tarivid, flobacin, and others. | [Manufacturing Process]
20 g of 2,3,4-trifluoronitrobenzene was dissolved in 150 ml of dimethyl sulfoxide, and to this mixture a solution of 10% potassium hydroxide was added dropwise while keeping the temperature at 18° to 20°C. Then, the mixture was stirred for 2 hours at room temperature and one liter of water was added to this reaction mixture and the mixture was shaken with chloroform. The water layer was acidified with hydrochloric acid and was extracted with chloroform. The extract was washed with water and was dried, then chloroform layer was concentrated. The residue was purified by silica gel column chromatography to provide 5.8 g of 2,3-difluoro-6-nitrophenol as yellow oil.
7.9 g of the 2,3-difluoro-6-nitrophenol, 50.1 g of 1,2-dibromoethane and 18.7 g of potassium carbonate were added to 80 ml of dimethylformamide and the mixture was stirred for 2.5 hours at from about 80° to 100°C (bath temperature). The reaction mixture was concentrated to dryness in vacuo and the residue was distributed between ethyl acetate and water. The organic solvent layer was washed with water and was dried, then the solvent was evaporated. The residue was dissolved in benzene and was purified by silica gel column chromatography to provide 7.7 g of 2-(2-bromoethoxy)-3,4difluoronitrobenzene as light yellow oil.
1.74 g of this product was dissolved in 30 ml of methanol and a solution of 6.44 g of sodium dithionite dissolved in 15 ml of water was added thereto. The mixture was stirred for 1 hour at room temperature. Methanol was evaporated and the residue was extracted with chloroform. After the extract was washed with water and dried, the solvent was evaporated to provide 0.44 g of 2-(2-bromoethoxy)-3,4-difluoroaniline.
1.82 g of this product and 3.03 g of potassium carbonate were added to 10 ml of dimethylformamide and the mixture was stirred for 1 hour at from about 80° to 100°C (bath temperature). The reaction mixture was added to ice-cold water and was extracted with ethyl acetate. After the extract was washed with water and dried, the solvent was distilled off at room temperature to provide 1.21 g of 7,8-difluoro-2,3-dihydro-4H-[1,4]benzoxazine with m.p. 48°-54°C.
The mixture of 1.1 g of this product and 1.38 g of diethyl
ethoxymethylenemalonate was stirred for 2 hours at from about 130° to 135°C (bath temperature). The ethanol produced was evaporated and 20 g of ethyl polyphosphate was added to the residue. Then the mixture was stirred for 1.5 hours at from about 140° to 145°C (bath temperature). The reaction mixture was added to ice-cold water and was extracted with chloroform. The extract was washed fully with water. After drying, the solvent was evaporated and the residue was recrystallized from ethyl acetate. 1.3 g of ethyl 9,10difluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6carboxylate was obtained as colorless needles with m.p. 265°-266°C.
1.15 g of this product was added to 12 ml of mixture of concentrated hydrochloric acid and acetic acid (1:4 by volume) and the mixture was stirred for 4 hours at 100° to 110°C (bath temperature). After cooling, the precipitated crystals were collected by filtration, washed with water, methanol and chloroform to give 0.78 g of 9,10-difluoro-7-oxo-2,3-dihydro-7Hpyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid as colorless needles with m.p. above 300°C.
1.0 g of 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3de][1,4]benzoxazine-6-carboxylic acid and 2.85 g of N-methylpiperazine were added to 15 ml of dimethylsulfoxide. The mixture was stirred at a temperature of from about 100° to 110°C (bath temperature) for 12 hours and the reaction mixture was concentrated to dryness in vacuo and 40 ml of water was added to the residue. Then the product was extracted with chloroform. The extract was dried and concentrated to dryness in vacuo. The residue was recrystallized from ethanol to provide 550 mg of 9-fluoro-3-methyl-10-(4methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3de][1,4]benzoxazine-6-carboxylic acid as colorless needles with m.p. 250°257°C (with decomposition).
| [Brand name]
Floxin (Ortho-McNeil); Floxin (Daiichi Pharmaceutical); Ocuflox (Allergan);TARIVID. | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
It exhibits good activity against a wide range of enterobacteria, including strains resistant to nalidixic acid, as well as against Aeromonas, Campylobacter, Vibrio and Moraxella spp. Activity against methicillin-sensitive Staph. aureus is good, but streptococci, including Str. pneumoniae and enterococci, are less susceptible. Most anaerobes are moderately or completely resistant. It is active against L. pneumophila, Ch. pneumoniae, C. trachomatis, mycoplasmas, ureaplasmas and M. tuberculosis. Other mycobacteria such as M. fortuitum, M. kansasii, M. chelonei and the M. avium complex are moderately susceptible.
| [Pharmaceutical Applications]
A tricyclic 6-fluoro, 7-piperazinyl quinoline with a methyl substituted oxazine ring substituted. It is a racemic mixture of l- (levofloxacin, see p. 319) and d-isomers. | [Biotechnological Applications]
Ofloxacin is a fluoroquinolone antibiotic present as a racemic mixture. Levofloxacin, S-isomer of ofloxacin, shows a broad spectrum of antibacterial activity against both gram-positive and gram-negative bacteria. The antibacterial activity of levofloxacin is 8–128 times greater than that of the corresponding R-isomer. A novel esterase of type B1 carboxylesterase/lipase family from a marine isolate Y. lipolytica CL180 was used to resolve a racemic mixture of ofloxacin ester. This esterase showed an enantioselectivity toward R, S-ofloxacin ester, and levofloxacin was produced with an enantiomeric excess of 52 % (Kim et al. 2007). | [Pharmacokinetics]
Oral absorption: c. 95%
Cmax400 mg oral: 3–5 mg/L after 1–1.5 h
200 mg intravenous (30-min infusion): 1.8 mg/L 1 h after end infusion
Plasma half-life: 5–7 h
Volume of distribution: 1–2.5 L/kg
Plasma protein binding: c. 25%
absorption and distribution
There is no significant interference with absorption by magnesium–aluminum hydroxide or calcium carbonate compounds,providing administration is separated by at least 2 h. In patients receiving repeated 200 mg doses, the mean peak plasma concentration rose from 2.7 mg/L after the first dose to 3.4 mg/L after the seventh.
It is widely distributed, achieving levels ≥50% of simultaneous plasma concentrations in many tissues, including lung and bronchial secretions. In cantharides and suction blisters, peak concentrations exceed those in plasma, while the elimination half-life is similar. In patients with non-inflamed meninges, 200 mg administered orally or by intravenous infusion over 30 min produced CSF concentrations of around 0.4–1 mg/L at 2–4 h while the plasma concentration was 1.7–4 mg/L: a 400 mg intravenous infusion yielded a CSF concentration of 2 mg/L, which is adequate for some Gram-negative bacteria, but not for Gram-positive bacteria or Ps. aeruginosa.
Metabolism and excretion
It is poorly metabolized into desmethyl and N-oxide derivatives (<5% of the administered dose), only about 20% of a dose being eliminated by non-renal routes. There is a very slight effect on cytochrome P450-related isoenzymes and no significant effect on the metabolism of theophylline in dosages of up to 800 mg.
About 60% of a dose appears in the urine over 12 h and 80–90% over 48 h. The apparent elimination half-life is prolonged in renal failure, reaching 30–50 h in anuria, necessitating a dosage reduction. The desmethyl metabolite accumulates in all patients and the N-oxide in 50%. Absorption and distribution are not affected by renal failure. Significant amounts of the drug appear in the feces, producing very variable
concentrations up to 100 mg/kg. | [Clinical Use]
9-Fluoro-2,3-dihydro-3-methyl-10(4-methyl-1-piperazin-yl)-7-oxo-7H-pyrido[1,2,3-de]-1,4,-benzoxazine-6-carboxylicacid (Floxin, Floxin IV) is a member of the quinolone class of antibacterial drugs wherein the 1- and 8-positions are joined in the form of a 1,4-oxazine ring.
Ofloxacin has been approved for the treatment of infectionsof the lower respiratory tract, including chronic bronchitisand pneumonia, caused by Gram-negative bacilli. It isalso used for the treatment of pelvic inflammatory diseaseand is highly active against both gonococci and chlamydia.In common with other fluoroquinolones, ofloxacin is not effectivein the treatment of syphilis. A single 400-mg oraldose of ofloxacin in combination with the tetracycline antibioticdoxycycline is recommended by the Centers forDisease Control and Prevention (CDC) for the outpatienttreatment of acute gonococcal urethritis. Ofloxacin is alsoused for the treatment of urinary tract infections caused byGram-negative bacilli and for prostatitis caused by E. coli.Infections of the skin and soft tissues caused by staphylococci,streptococci, and Gram-negative bacilli may also betreated with ofloxacin. | [Clinical Use]
Complicated and uncomplicated infections of the urinary tract, chronic prostatitis
Uncomplicated urogenital and anorectal gonorrhea (single-dose), chancroid (3-day course), genital chlamydial infections (7-day course) Lower respiratory tract infections, including bronchopneumonia, community-acquired pneumonia (except pneumococcal pneumonia), acute bacterial exacerbations of chronic bronchitis (unless pneumococci are involved) and bronchiectasis
Enteric fever, including the chronic carrier state; gastroenteritis caused by enterotoxigenic Escherichia coli, Salmonella, Shigella and Campylobacter spp. Ocular infections (ophthalmic preparation) | [Side effects]
Untoward reactions havebeen described in 2.5–7.5% of patients, and are those common to the group: gastrointestinal tract disturbances, rashes, tendon rupture and insomnia. CNS effects rarely occur. | [Safety Profile]
Poison by intravenous route.Moderately toxic by ingestion. An experimental teratogen.Other experimental reproductive effects. Human systemiceffects: body temperature increase, diarrhea,hallucinations, hypermotility, irritability, psychosis.Mutation d | [Synthesis]
Ofloxacin is synthesized from 2,3,4-trifluoronitrobenzene, which
upon reaction with potassium hydroxide gives 2-hydroxy-3,4-difluoronitrobenzene
(33.2.25). Reacting this with chloroacetone in the presence of potassium iodide and
potassium carbonate gives the corresponding ether of hydroxyacetone (33.2.26).
Exhaustive reduction of this compound with hydrogen over Raney nickel in one step
gives the desired derivative of difluorobenzoxazine (33.2.27), bypassing, perhaps unnec�essary, if not impossible, isolation stages of the amine, then the internal imine, and finally
the desired product. According to the schemes of synthesis that have been repeated many
times above, the secondary heterocyclic amine (33.2.27) is reacted with ethyl
ethoxymethylenmalonate, and the resulting aminomethylmalonic derivative (33.2.28)
cyclizes into a pyrido-benzoxazine system. However, unlike any of the cases described
above where the reaction was done at high temperatures, this reaction is accomplished
using polyphosphoric acid. The resulting ethyl ester of 9,10-difluoro-3-methyl-7H�pyrido (1,2,3-de)-1,4-benzoxazin-7-oxo-6-carboxylic acid undergoes hydrolysis to the corresponding acid (33.2.29). Finally, reacting this product with N-methylpiperazine
replaces the fluorine atom at position C10 of the pyridobenzoxazine system, forming
orloxacin (32.2.30).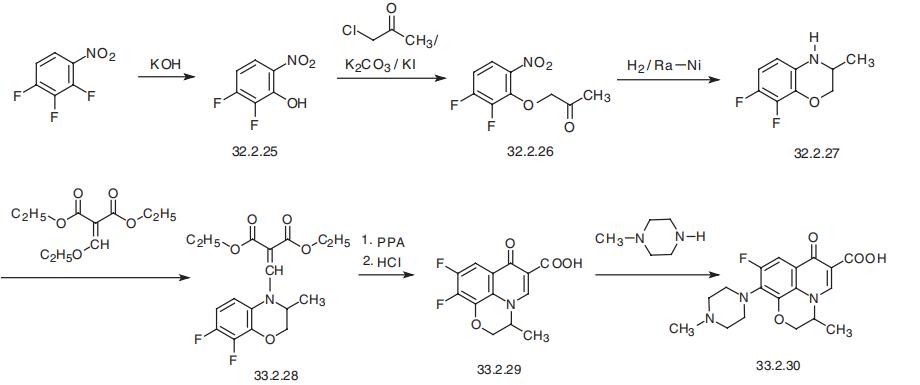
| [Drug interactions]
Potentially hazardous interactions with other drugs
Aminophylline: possibly increased risk of
convulsions, increased levels of aminophylline.
Analgesics: increased risk of convulsions with
NSAIDs.
Anticoagulants: anticoagulant effect of coumarins
enhanced.
Antimalarials: manufacturer of artemether with
lumefantrine advises avoid.
Ciclosporin: increased risk of nephrotoxicity.
Theophylline: possibly increased risk of convulsions. | [Metabolism]
Ofloxacin undergoes limited metabolism to desmethyl
and N-oxide metabolites; desmethylofloxacin has
moderate antibacterial activity.
Excretion is by tubular secretion and glomerular
filtration, and 65-80% of a dose is excreted unchanged
in the urine over 24-48 hours, resulting in high urinary
concentrations.
Less than 5% is excreted in the urine as metabolites. From
4-8% of a dose may be excreted in the faeces. |
|
|