| Identification | Back Directory | [Name]
1-[[(6R,7R)-7-[[(2Z)-(2-Amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino] acetyl] amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-en-3-yl]methyl]pyridinum hydroxide inner salt | [CAS]
72558-82-8 | [Synonyms]
hy
caz
sn401
Ceptaz
Fortum
Biotum
Fortaz
Fortam
Panzid
C06889
Stareef
Kefadim
Kefamin
Tazicef
Modacin
gr20263
Tazidime
Glazidim
thermogra
Ceftacidin
72558-82-8
CEFTAZIDINE
Ceftazidime
Tazicef:CAZ
Ceftim�����,F(xiàn)ortam
CEFTAZIDIME HYDRATE
Ceftazidime/Sodium Carbonate
CeftazidiMe (C22H22N6O7S2 · xH2O)
CeftazidiMe with SodiuM Carbonate
CeftazidimePentahydrateBufferedUsp24
Ceftazidime (contains ca. 10% Na2CO3)
innersalt,(6r-(6-alpha,7-beta-(z)))-droxid
pyridinium,1-((7-(((2-amino-4-thiazolyl)((1-carboxy-1-methylethoxy)imino)acety
(6R,7R)-7-[(Z)-2-(Aminothiazol-4-yl)-2-(2-carboxypropoxyimino)acetamido]-3-(1-pyridiniummethyl)ceph-3-em-4-carboxylate
(6R,7R)-7-[[(Z)-(2-Amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-8-oxo-3-(pyridiniomethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
(6R,7R)-7-[2-(2-Amino-4-thiazolyl)-2-[(Z)-1-carboxy-1-methylethoxyimino]acetylamino]-8-oxo-3-(pyridiniomethyl)-5-thia-1-azabicyclo[4.2.0]octan-2-ene-2-carboxylate
[(6R-[6α,7β(Z)]]-1-[[7-[[(2-Amino-4-thiazolyl)[(1-earboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabieyelo[4.2.0]oct-2-en-3-y1]methyl]pyridinium inner salt
Pyridinium, 1-[[(6R,7R)-7-[[(2Z)-(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, inner salt
1-[[(6R,7R)-7-[[(2Z)-2-(2-Amino-4-thiazolyl)-2-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinium Inner Salt
1-[[(6r,7r)-7-[[(2z)-(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino] acetyl] amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-en-3-yl]methyl]pyridinum hydroxide inner salt
Pyridinium, 1-[[7-[[(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]- | [EINECS(EC#)]
276-715-9 | [Molecular Formula]
C22H22N6O7S2 | [MDL Number]
MFCD00072034 | [MOL File]
72558-82-8.mol | [Molecular Weight]
546.58 |
| Chemical Properties | Back Directory | [Appearance]
solid | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
≥21.25 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O | [form ]
powder to crystal | [color ]
White to Orange to Green | [Stability:]
Stable, but keep refrigerated. Incompatible with strong oxidizing agents, nitric acid, permanganates, peroxides. | [Merck ]
14,1946 |
| Hazard Information | Back Directory | [Chemical Properties]
solid | [Usage]
5-HT agonist, anti-migrane | [Usage]
pyrimidine synthesis inhibitor disease-modifying antirheumatic drug | [Usage]
Third generation cephalosporin antibiotic. Antibacterial | [Description]
In ceftazidime the oxime moiety is more complex, containing two methyl groups and a carboxylic acid. This
assemblage conveys even more pronounced β-lactamase stability, greater anti–Pseudomonas aerugi nosa,
and increased activity against Gram-positive organisms. The C-3 side chain has been replaced by a charged
pyridinium moiety. The latter considerably enhances water solubility and also highly activates the β-lactam
bond toward cleavage. The drug must be protected against heat and light and may darken without significant
loss of potency. It is not stable under some conditions. such as the presence of aminoglycosides and
vancomycin. It also is attacked readily in sodium bicarbonate solutions. Resistance is mediated by
chromosomally mediated β-lactamases and by lack of penetration into target bacteria. Otherwise, it has a
very broad antibacterial spectrum. | [Originator]
Fortum,Glaxo,UK,1983 | [Uses]
5-HT agonist, anti-migrane | [Uses]
Like most of the third-generation cephalosporin antibiotics described above, ceftazidime
has a broad spectrum of antimicrobial action, including the most clinically important
microorganisms: Gram-positive, Gram-negative, aerobic, and anaerobic. It is resistant to
most beta-lactamases of Gram-positive and Gram-negative bacteria.
It is used for treating most serious bacterial infections. Synonyms of this drug are for�tum, ceftim, stacef, and tazicef. | [Uses]
pyrimidine synthesis inhibitor disease-modifying antirheumatic drug | [Uses]
Third generation cephalosporin antibiotic. Antibacterial | [Definition]
ChEBI: A cephalosporin bearing pyridinium-1-ylmethyl and {[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. | [Manufacturing Process]
(a) t-Butyl(6R,7R)-3-acetoxymethyl-7-[(Z)-2-(2-t-butoxycarbonylprop-2-
oxyimino)-2-(2-tritylaminothiazol-4-yl)acetamido]ceph-3-em-4-carboxylate: A
stirred solution of (Z)-2-(2-t-butoxycarbonylprop-2-oxyimino)-2-(2-
tritylaminothiazol-4-yl)acetic acid (572 mg) and t-butyl(6R,7R)-3-
acetoxymethyl-7-aminoceph-3-em-4-carboxylate (328 mg) in
dimethylformamide (10 ml) was cooled to 0°C, and 1-hydroxybenzotriazole
(150 mg) was added, followed by dicyclohexylcarbodiimide (225 mg). The
mixture was warmed to room temperature, stirred for 5 hours and allowed to
stand overnight. The mixture was filtered, and the white solid washed with a
little ether. The filtrate and washings were diluted with water (50 ml) and
extracted with ethyl acetate. The organic extracts were combined, washed
successively with water, 2 N hydrochloric acid, water, sodium bicarbonate
solution, and saturated brine, dried and evaporated. The residue was eluted
through a silica column with ether. The product-containing eluate was
collected and concentrated to give the title compound (533 mg). A portion
was recrystallized from diisopropyl ether, MP 103°C to 113°C (decomp.);
[α]D20 +8.5 (conc. 1.0, DMSO).
(b) (6R,7R)-3-Acetoxymethyl-7-[(Z)-2-(2-aminothiazol-4-yl)-2-(2-
carboxyprop-2-oxyimino)acetamido]ceph-3-em-4-carboxylic acid:
Trifluoroacetic acid (18 ml) was added to a solution of the product of (a) (2.4
g) in anisole (18 ml) at 0°C. The mixture was stirred at room temperature for
2 hours and concentrated. The residue was dissolved in ethyl acetate and
extracted with saturated sodium bicarbonate solution. The pH of the aqueous
extracts was adjusted to 6, and the solution washed with ethyl acetate. The
aqueous phase was acidified to pH 1.5 under ethyl acetate, saturated with
sodium chloride, and extracted with ethyl acetate. The combined organic
extracts were washed with saturated brine, dried and evaporated. The residue
was dissolved in warm 50% aqueous formic acid (20 ml) and allowed to stand
for 2 hours. The mixture was diluted with water (50 ml) and filtered. The
filtrate was concentrated. The residue was taken up in water (50 ml),
refiltered, and lyophilized to give the title compound (920 mg).
(c) (6R,7R)-7-[(Z)-(2-Aminothiazol-4-yl)-2-(2-carboxyprop-2-
oxyimino)acetamido]-3-(1-pyridiniummethyl)-ceph-3-em-4-carboxylate,
monosodium salt: Pyridine (2 ml) and the product of (b) (1.8 g) were added
to a stirred solution of sodium iodide (7.12 g) in water (2.2 ml) at 80°C. The
solution was stirred at 80 C for 1 hour, cooled, and diluted to 100 ml with
water. The pH of the solution was adjusted to 6.0 with 2N sodium hydroxide
solution, and this solution was concentrated to remove pyridine. The aqueous
residue was diluted to 100 ml with water, methyl isobutyl ketone (2 drops) was added, and the solution was acidified to pH 1 with 2 N hydrochloric acid.
The mixture was filtered, and the solid was washed with a little water. The
filtrate and washings were collected and washed with ethyl acetate, and the
pH adjusted to 6.0 with 2 N sodium hydroxide solution. The solution was
concentrated to 50 ml and applied to a column of 500 g Amberlite XAD-2
resin, using first water and then 20% aqueous ethanol as eluting solvent. The
product-containing fractions were concentrated and lyophilized to give the title
compound (0.56 g). | [Brand name]
Fortaz
(GlaxoSmithKline); Tazicef (Hospira); Tazidime (Lilly). | [Therapeutic Function]
Antibiotic | [Clinical Use]
Antibacterial agent | [Synthesis]
Ceftazidime is 1-[[7-[[(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)
imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-
yl]methyl]pyridin-2-carboxylic acid (32.1.2.82). As is the case in synthesis of ceftriazone,
the synthesis of ceftazidime requires the preliminary synthesis of two starting com�pounds. 7-Amino-3-(1-pyridinomethyl)cef-3-en-carboxylic acid dihydrochloride is used as the cephalosporin fragment, while the acyl fragment is a modified structure of
(32.1.2.77), which is not a derivative of 2-(2-amino-4-thiazolyl)-2-methoxyminoacetic
acid, but a derivative of 2-(2-amino-4-thiazolyl)-2-(2-tert-butoxycarboxyl-2-propylox�imino)acetic acid, which is synthesized by the following scheme. Nitration of ace�toacetic ester gives isonitrosoacetoacetic ester (32.1.2.49), which undergoes chlorination
by sulfuryl chloride in methylene chloride to form 4-chloro-2-hydroximinoacetoacetic
ester (32.1.2.73).
Reacting this with thiourea in the classic scheme of thiazole synthesis by reacting of
|á-halogencarbonyl compounds with thioamides forms the ethyl ester of (Z)-2-(2-aminoth�iazole-4-yl)-2-hydroxyminoacetic acid (32.1.2.74). The amino group in this molecule is
protected by a reaction with triphenylchloromethane in dimethylformamide in the pres�ence of triethylamine, which gives the ethyl ester of (Z)-2-(2-tritylaminothiazole-4-yl)-
2-hydroxyminoacetic acid (32.2.3.75). The hydroxyl group in the resulting compound is
alkylated with the tert-butyl ester of |á-bromoisobutyric acid in dimethylsulfoxide in the
presence of potassium carbonate, giving ethyl ester of 4-thiazoleacetic acid,
|á -[[2-(1,1-dimethylethoxy)-1,1-dimethyl-2-oxoethoxy]imino]-2-
[(triphenylmethyl)amino], (Z) (32.1.2.76). The ethoxycarbonyl group in this molecule is
hydrolyzed by sodium hydroxide, and upon working up the reaction mixture with an acid,
the corresponding acid (32.1.2.77) is isolated (32.1.2.77). Upon interaction with phospho�rous pentachloride the acid chloride (32.1.2.78) is obtained, which is used further as the
acylating reagent.
The second necessary fragment, 7-amino-3-(1-pyridinomethyl)cef-3-en-carbonic acid
(32.1.2.80), is synthesized from cefalosporidin (32.1.2.79), a cephalosporin antibiotic that
is used independently in medicine and which is synthesized in the form of an internal salt
by reacting cefalotin (32.1.2.1) with pyridine to replace the acetoxyl group with a pyridine
group. Initially treating cephaloridin with trimethylchlorosilane in the presence of
dimethylaniline and then with phosphorous pentachloride, followed by a reaction with 1,
3-butandiol results in the creation of 7-amino-3-(1-pyridinomethyl)cef-3-en-carboxylic
acid (32.1.2.80). This is acylated by the acid chloride (32.1.2.78) synthesized earlier, form�ing the product (32.1.2.81), which is treated with a mixture of formic and hydrochloric
acids to remove both protective groups (triphenylmethyl and tert-butyl), giving cef�tazidime (32.1.2.82) in the form of a dihydrochloride.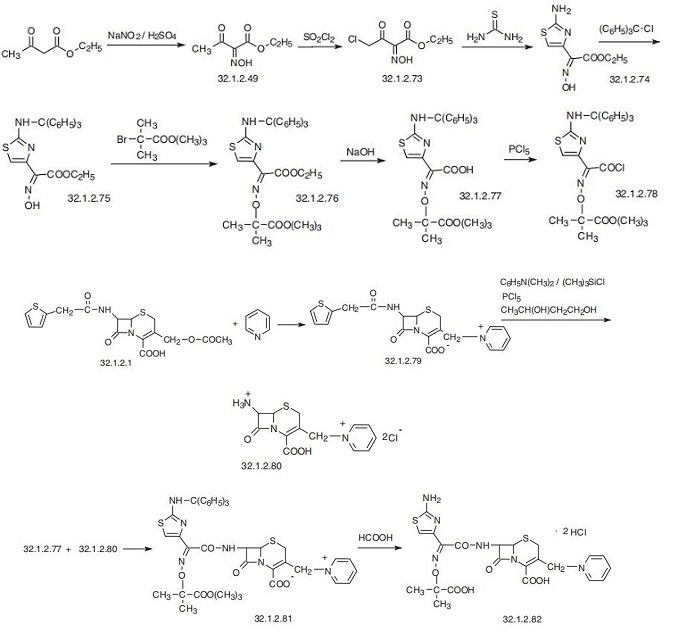
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced.
Ciclosporin: may cause increased ciclosporin levels. | [Metabolism]
Ceftazidime is passively excreted in bile, although only
a small proportion (1%) is eliminated by this route. It
is mainly excreted by the kidneys, almost exclusively by
glomerular filtration; probenecid has little effect on the
excretion. About 80-90% of a dose appears unchanged in
the urine within 24 hours. |
|
|