| Identification | More | [Name]
Ceftizoxime | [CAS]
68401-81-0 | [Synonyms]
CEFTIZOXIME
CEFTIZOXIME ACID
EPOCELIN
(6r-(6-alpha,7-beta(z)))-l)(methoxyimino)acetyl)amino)-8-oxo
5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-(((2-amino-4-thiazoly
7-Amino-3-[[[2,5-Dihydro-6-Hydroxy-2-Methyl-5-Oxo-1,2,4-Trizin-3-Yl]-Thio]Methyl]-Cephalosporanic Acid
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-, [6R-[6α,7β(Z)]]-
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-, (6R,7R)-
Ceftisomin
syn-7-(2-(2-amino-4-thiazolyl)-2-methoxyiminoacetamido)-3-cephem-4-carbo xyli
(6R,7R)-7-[[(2Z)-(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[(6R-[6α����,7β(Z)])-7-[[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct2-ene-2-carboxylie acid
Cefimx
FR-13749
(7R)-7-[[2-(2-Aminothiazole-4-yl)-2-(methoxyimino)-1-oxoethyl]amino]cepham-3-ene-4-carboxylic acid
(7R)-7β-[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetylamino]cepham-3-ene-4-carboxylic acid
(6R,7R)-7-[(Z)-2-(2-Amino-4-thiazolyl)-2-methoxyiminoacetylamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
(6R,7R)-7α-[2-(2-Imino-4-thiazolin-4-yl)-2-[(Z)-methoxyimino]acetylamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Ceftizox | [EINECS(EC#)]
629-729-8 | [Molecular Formula]
C16H17N5O8S | [MDL Number]
MFCD00072000 | [Molecular Weight]
439.4 | [MOL File]
68401-81-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
227° (dec) | [density ]
1.89±0.1 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [solubility ]
Aqueous Base (Slightly), DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated) | [form ]
Solid | [pka]
pKa 2.1 (Uncertain) | [color ]
White to Pale Yellow | [Merck ]
14,1951 | [Stability:]
Hygroscopic | [InChIKey]
NNULBSISHYWZJU-LLKWHZGFSA-N | [SMILES]
N12[C@@]([H])([C@H](NC(/C(/C3=CSC(N)=N3)=N\OC)=O)C1=O)SCC=C2C(O)=O | [CAS DataBase Reference]
68401-81-0(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
In ceftizoxime, the whole C-3 side chain has been omitted to prevent deactivation by hydrolysis. It rather
resembles cefotaxime in its properties; however, not being subject to metabolism, its pharmacokinetic
properties are much less complex. | [Originator]
Eposelin,Fujisawa,Japan,1982 | [Uses]
Antibacterial. | [Uses]
Ceftizoxime is a cephalosporin based, potent antibacterial agent. | [Uses]
Ceftizoxime is used for bacterial infections of the lower respiratory tract, infections of the
urinary tract, infections of the bones, joints, skin, soft tissues, and abdominal infections.
Synonyms of this drug are ceftix and eposerin. | [Definition]
ChEBI: A parenteral third-generation cephalosporin, bearing a 2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino group at the 7beta-position. | [Manufacturing Process]
Phosphorus oxychloride (2.0 g) was added at one time at 5°C to 10°C to a
suspension of 2-methoxyimino-2-(2-amino-1,3-thiazol-4-yl)acetic acid (syn
isomer) (2 g) in dry ethyl acetate (20 ml). After stirring for 20 minutes at 7°C
to 10°C, bis(trimethylsilyl)acetamide (0.4 g) was added thereto at the same
temperature. After stirring for 10 minutes at 7°C to 10°C, phosphorus
oxychloride (2.0 g) was dropwise added thereto at the same temperature. The
resulting mixture was stirred for 10 minutes at 7°C to 10°C, and dry
dimethylformamide (0.8 g) was dropwise added thereto at the same
temperature. The mixture was stirred for 30 minutes at 7°C to 10°C to give a
clear solution. On the other hand, trimethylsilylacetamide (7.35 g) was added
to a suspension of 7-aminocephalosporanic acid (2.45 g) in dry ethyl acetate
(8 ml), after which the mixture was stirred at 40°C to give a clear solution.
To this solution was added at one time the above-obtained ethyl acetate
solution at -15°C, and the resulting mixture was stirred for 1 hour at -10°C to
-15°C. The reaction mixture was cooled to -30°C, and water (80 ml) was
added thereto. The aqueous layer was separated, adjusted to pH 4.5 with
sodium bicarbonate and subjected to column chromatography on Diaion HP-20
resin (Mitsubishi Chemical Industries Ltd.) using 25% aqueous solution of
isopropyl alcohol as an eluent. The eluate was lyophilized to give 7-[2-
methoxyimino-2-(2-amino-1,3-thiazol-4-yl)acetamido]cephalosporanic acid
(syn isomer) (1.8 g), MP 227°C (decomp.). | [Brand name]
Cefizox (Astellas). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
A semisynthetic cephalosporin supplied as the sodium salt. The
properties are very similar to those of cefotaxime, but it lacks
the acetoxymethyl group at position C-4 and is therefore not
subject to deacetylation. Activity against common pathogenic
bacteria (Table 13.4) is very similar to that of cefotaxime.
A 500 mg intramuscular injection achieves a plasma concentration
of around 14 mg/L. A concentration of 85–90 mg/L
is produced 30 min at the end of a 30-min intravenous infusion.
The plasma half-life is 1.3–1.9 h. Protein binding is
30%. It is well distributed. In children with meningitis receiving
200–250 mg/kg per day in four equally divided doses for
14–21 days, mean CSF concentrations 2 h after a dose were
6.4 mg/L on day 2 and 3.6 mg/L on day 14.
About 70–90% of the dose is recovered in the urine in the first
24 h, principally by glomerular filtration. Probenecid increases
the plasma half-life by about 50%. In patients receiving 1 g
intravenously over 30 min, the plasma elimination half-life rose
to 35 h when the corrected creatinine clearance was <10 mL/
min. It is partly removed by peritoneal and hemodialysis.
Adverse reactions and clinical use are similar to those of
cefotaxime. | [Synthesis]
Ceftizoxime, |á-O-methyloxime of (6R,7R)-7-[2-(2-amino-4-thiazolyl)glyoxy�lamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.64), is synthe�sized by the scheme described below, which begins with 4-nitrobenzyl ester of
3-hydroxy-7-(2-phenylacetamido)-3-cefem-4-carboxylic acid (32.1.2.57), which is synthe�sized using a number of methods used to synthesize cefaclor (32.1.2.48). Reducing the C3¨CC4
double bond in the initial 4-nitrobenzyl ester of 3-hydroxy-7-(2-phenylacetamido)-3-cefem-
4-carboxylic acid (32.1.2.57) with sodium borohydride in methanol, 4-nitrobenzyl ester of
3-hydroxy-7-(2-phenylacetamido)-3-cefam-4-carboxylic acid (32.1.2.58) is obtained, the
hydroxyl group in which it is acylated by acetic anhydride in pyridine, forming acetate
(32.1.2.59). Reacting this with triethylamine removes a molecule of acetic acid, giving the
4-nitrobenzyl ester of 7-(2-phenylacetamido)-3-cefem-4-carboxylic acid (32.1.2.60).
Reacting this with phosphorous pentachloride in pyridine, followed by subsequent methanol�ysis deacylates the amide fragment of the molecule, giving the 4-nitrobenzyl ester of 7-amino-
3-cefem-4-carboxylic acid (32.1.2.61).
Preliminary silylation of the amino group of this
compound with trimethylsilylacetamide and subsequent acylation with 2-(2-formamido-4-thi�azolyl)-2-methoxyminoacetic acid chloride synthesized directly in reaction conditions by
reacting with phosphorous chloroxide in dimethylformamide gives the 4-nitro-benzyl ester of
|á-O-methyloxime of 7-[2-(2-formamido-4-thiazolyl)glyoxylamido]-8-oxo-t-thia-1-azabicy�clo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.62). Reducing this with hydrogen using a palla�dium on carbon catalyst removes the 4-nitrobenzyl protection from the carboxyl group,
forming the acid (32.1.2.63). Finally, hydrolysis of the formamide region of the molecule
using hydrogen chloride in methanol gives the desired ceftizoxime (32.1.2.64).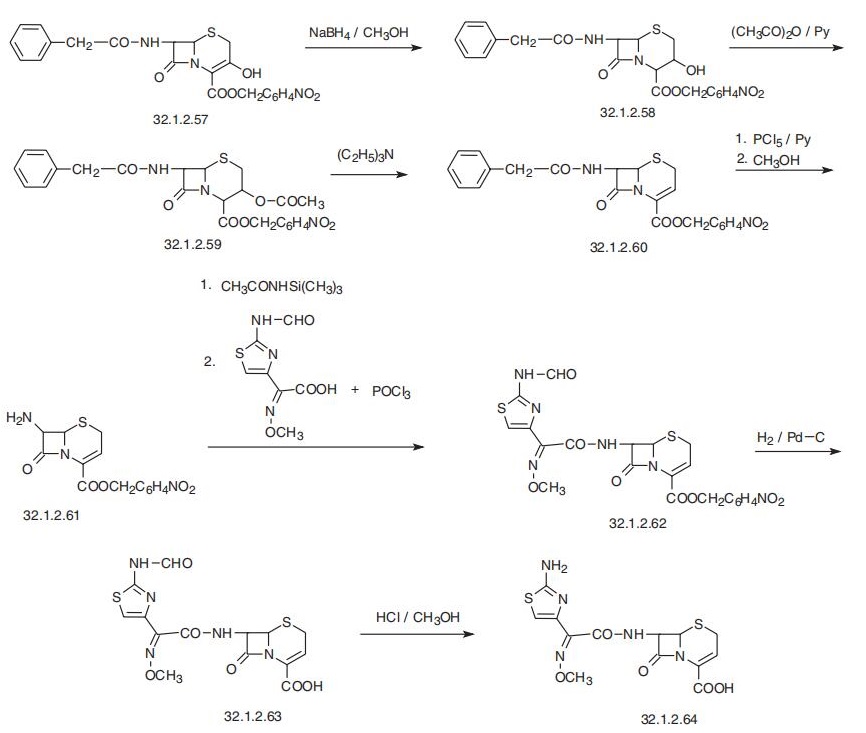
| [storage]
4°C, away from moisture and light |
|
|