| Identification | More | [Name]
Mitoxantrone | [CAS]
65271-80-9 | [Synonyms]
1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]-anthracene-9,10-dione
MITOXANTRONE
1,4-dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthracenedi
5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxy-anthraquinon
5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxyanthraquinone
9,10-anthracenedione,1,4-dihydroxy-5,8-bis((2-((2-hydroxyethyl)amino)ethyl)a
dihydroxyanthraquinone
mitoxanthrone
nsc279836
MitoxantroneBase
Mitoxantrone Hcl 70476-82-3/Base
PharmasubstanceEP4
1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione
NSC-27983
9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-
1,4-dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthrace nedimitoxantrone
MITOXANTRONUM AND THE INTERMEDIATES
Mitoxantrone (base and/or unspecified salts)
MITOXANTRONUM
1,4-Dihydroxy-5,8-bis[2-[(2-hydroxyethyl)amino]ethylamino]-9,10-anthraquinone | [EINECS(EC#)]
1533716-785-6 | [Molecular Formula]
C22H28N4O6 | [MDL Number]
MFCD00242942 | [Molecular Weight]
444.48 | [MOL File]
65271-80-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Dark Blue Crystalline Solid | [Melting point ]
170-1740C | [Boiling point ]
554.47°C (rough estimate) | [density ]
1.3049 (rough estimate) | [refractive index ]
1.6500 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Sparingly), Water (Slightly) | [form ]
Solid | [pka]
pKa 5.99 (Uncertain);8.13 (Uncertain) | [color ]
Dark Blue to Black | [Usage]
A DNA intercalating drug. Inhibits DNA synthesis. Used as an anti-cancer agent | [InChI]
InChI=1S/C22H28N4O6/c27-11-9-23-5-7-25-13-1-2-14(26-8-6-24-10-12-28)18-17(13)21(31)19-15(29)3-4-16(30)20(19)22(18)32/h1-4,23-30H,5-12H2 | [InChIKey]
KKZJGLLVHKMTCM-UHFFFAOYSA-N | [SMILES]
C1(O)=C2C(C(=O)C3=C(C2=O)C(NCCNCCO)=CC=C3NCCNCCO)=C(O)C=C1 | [CAS DataBase Reference]
65271-80-9(CAS DataBase Reference) | [IARC]
2B (Vol. 76) 2000 |
| Safety Data | Back Directory | [Hazard Codes ]
T,T+ | [Risk Statements ]
R61:May cause harm to the unborn child.
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S22:Do not breathe dust . | [WGK Germany ]
3
| [RTECS ]
CB5748500
| [HS Code ]
2922.50.2500 |
| Hazard Information | Back Directory | [Chemical Properties]
Dark Blue Crystalline Solid | [Originator]
Novantrone,Immunex Corporation | [Uses]
A DNA intercalating drug. Inhibits DNA synthesis. Used as an anti-cancer agent | [Uses]
Mitoxantrone is a DNA intercalating drug. Mitoxantrone inhibits DNA synthesis. Mitoxantrone is used as an anti-cancer agent. | [Definition]
ChEBI: Mitoxantrone is a dihydroxyanthraquinone that is 1,4-dihydroxy-9,10-anthraquinone which is substituted by 6-hydroxy-1,4-diazahexyl groups at positions 5 and 8. It has a role as an antineoplastic agent and an analgesic. | [Indications]
Mitoxantrone (Novantrone) is a synthetic anthraquinone
that is structurally and mechanistically related to the anthracyclines.
It intercalates with DNA and produces single-
strand DNA breakage. It is cross-resistant with doxorubicin
in multidrug-resistant cells and in patients who
have failed to respond to doxorubicin therapy.
Mitoxantrone is active against breast carcinomas,
leukemias, and lymphomas. Its antitumor efficacy in patients
with breast cancer is slightly lower than that of
doxorubicin. Its major toxicity is myelosuppression; mucositis
and diarrhea also may occur. Mitoxantrone produces
less nausea, alopecia, and cardiac toxicity than
does doxorubicin. | [Manufacturing Process]
A suspension of 12.5 g of 2-(2-aminoethylamino)ethanol in 40 ml of
N,N,N',N'-tetramethylethylenediamine is stirred and de-aerated by bubbling
nitrogen in for 15 min. A 10.97 g of leuco-1,4,5,8-tetrahydroxyanthraquinone
is gradually added with stirring. The suspension is heated and stirred under
nitrogen at 50-52°C for 5 hours. The mixture is allowed to stand and cool
under nitrogen for 12 hours. The solid is collected by decantation, macerated
in ethanol, collected and washed with ethanol giving 15.06 g of the desired
product leuco-1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-
dihydroxyanthraquinone as a green-gray solid, melting point 129-131°C.
Chloranil oxidation. To 17.86 g of a suspension of the leuco-1,4-bis[2-(2-
hydroxyethylamino)ethylamino]-5,8-dihydroxyanthraquinone (0.03 mole) in 2-
methoxyethanol was added gradually with stirring 15 ml of 8 N ethanolic
hydrogen chloride. The system was chilled with an ice bath and stirred as 7.50
g (0.0305 mole) of chloranil powder was gradually added. The mixture was
stirred overnight at room temperature and diluted with 600 ml of ether. The
solid was collected and washed with tetrahydrofuran. Yield of 1,4-bis[2-(2-
hydroxyethylamino)ethylamino]-5,8-dihydroxyanthraquinone dihydrochloride
21.34 g, melting point 203-205°C (without recrystallisation). | [Brand name]
Novantrone (Serono). | [Therapeutic Function]
Antineoplastic | [Mechanism of action]
The mechanism of its action is not completely understood, although it is presumed, that
mitoxantrone acts by binding with DNA, thus disturbing the twisting process of the chains.
It is used intravenously for treating severe nonlymphatic leukemia, breast cancer, and so
on. A synonym of this drug is novantrone. | [Clinical Use]
#N/A | [Synthesis]
Mitoxantrone, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl) amino)
ethyl]amino]]-9,10-anthracendione (30.6.3), is structurally related to the antibiotic dox�orubicine. It is synthesized from danthron (1,8-dihydroxyanthraquinone), which when
reacted with nitric acid, and then a mixture of sodium sulfide and thiosulfate in a base, is
transformed to 1,4,5,8-tetrahydroxyanthraquinone (30.6.2). Reacting this with 2-amino�ethylaminoethanol in the presence of chloranyl (2,3,5,6-tetrachlorobenzoquinone-1,4)
gives the desired mitoxantrone (30.6.3),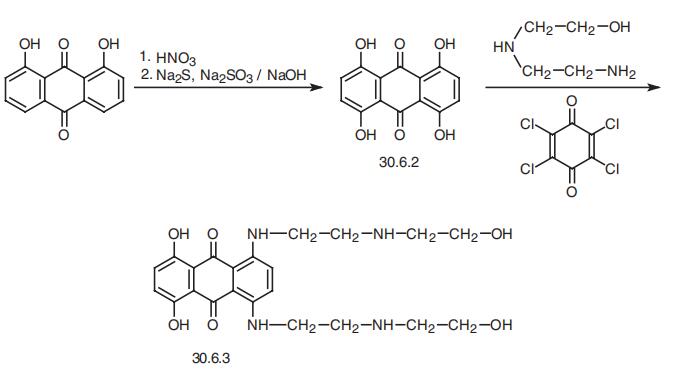
| [Veterinary Drugs and Treatments]
Mitoxantrone may be useful in the treatment of several neoplastic
diseases in dogs and cats, including lymphosarcoma mammary
adenocarcinoma, squamous cell carcinoma, renal adenocarcinoma,
fibroid sarcoma, thyroid or transitional cell carcinomas, and hemangiopericytoma.
Because renal clearance of the drug is minimal (10%), it may be
administered to cats with renal insufficiency much more safely than
doxorubicin. | [Drug interactions]
Potentially hazardous interactions with other drugs
Other antineoplastic agents: enhanced
myelosuppression - when used in combination
reduce mitoxantrone dose by 2-4 mg/m2.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Cardiotoxic drugs: increased risk of cardiac toxicity.
Ciclosporin: excretion of mitoxantrone reduced.
Live vaccines: risk of generalised infections - avoid. | [Metabolism]
Extensive metabolism in the liver.
Excretion is predominantly via the bile and faeces. 5-10%
of a dose is excreted in the urine and 13-25% in the
faeces, within 5 days | [storage]
-20°C, protect from light |
|
|