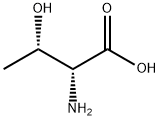
| Identification | More | [Name]
D-Threonine | [CAS]
632-20-2 | [Synonyms]
(2R,3S)-2-AMINO-3-HYDROXYBUTYRIC ACID
D-2-AMINO-3-HYDROXYBUTANOIC ACID
D-2-AMINO-3-HYDROXYBUTYRIC ACID
D-A-AMINO-B-HYDROXYBUTYRIC ACID
D-ALPHA-AMINO-BETA-HYDROXYBUTYRIC ACID
D-(+)-THREONINE
D-THREONINE
D-THREONINE, ALLO-FREE
H-D-THR-OH
(r)-threonine
d-threonin
D-α-Amino-β-hydroxybutyric acid
D-THREONINE, 98% (99% EE/GLC)
D-THREONINE, FREE OF ALLOTHREONINE
D-Threonine,>99%
D-THREONINE extrapure
(2R,3S)-(+)-Threonine, (2R,3S)-2-Amino-3-hydroxybutyric acid, D-α-Amino-β-hydroxybutyric acid
(2R,3S)-2-Amino-3-hydroxybutyric acid, D-α-Amino-β-hydroxybutyric acid | [EINECS(EC#)]
211-171-8 | [Molecular Formula]
C4H9NO3 | [MDL Number]
MFCD00064269 | [Molecular Weight]
119.12 | [MOL File]
632-20-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder or crystals | [Melting point ]
274 °C
| [alpha ]
28 º (c=6, water) | [Boiling point ]
222.38°C (rough estimate) | [density ]
1.3126 (rough estimate) | [refractive index ]
28 ° (C=3, H2O) | [storage temp. ]
Store at RT. | [solubility ]
H2O: soluble | [form ]
Crystals or Crystalline Powder | [pka]
2.19±0.10(Predicted) | [color ]
White | [Water Solubility ]
soluble | [Merck ]
9380 | [BRN ]
1721643 | [InChIKey]
AYFVYJQAPQTCCC-STHAYSLISA-N | [CAS DataBase Reference]
632-20-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
XO8580000
| [HS Code ]
29225000 |
| Questions and Answers (Q&A) | Back Directory | [Description]
D-threonine (632-20-2) is the D-form of threonine, which is a kind of amino acids. It can be used for the preparation of enantiomerically pure 1, 3-butanediol. It is also used as the intermediate during the preparation of chiral compounds such as antibiotics. D-threonine is an effective chiral pool reagent which contains an extra stereo-center. For example, people has recently used it as the starting material for total synthesis of protected legionaminic acid.
| [References]
https://www.alfa.com/zh-cn/catalog/B21177/
Ikemi, Masahisa, et al. "D-Threonine Aldolase and Its Application to D -β-Hydroxy-£-Amino Acid Synthesis." (1992).
Paek SM, et al. "Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis." Molecules 21.7(2016):951.
|
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder or crystals | [Uses]
A steroisomer of the proteinogenic amino acid L-threonine. | [Application]
D-Threonine is a steroisomer of the proteinogenic amino acid L-threonine. D-threonine is an important organic chiral source, mainly used in the fields of chiral drugs, chiral additives, chiral auxiliaries, etc., as a chiral source for chiral synthesis in the pharmaceutical industry. As an optically active organic acid, it has an irreplaceable role in the asymmetric synthesis of certain chiral compounds. It is mainly used to produce new broad-spectrum antibiotics, D-threonine, and threonine protection in peptide synthesis. | [Definition]
ChEBI: An optically active form of threonine having D-configuration. | [benefits]
Threonine, pronounced three-uh-neen is one of nine essential amino acids your body needs to function properly. Aiding in maintaining healthy skin, teeth, collagen, elastin, and muscle tissue, it also helps with digestion, metabolism and preventing fat buildup in the liver. Threonine has been used to treat intestinal disorders and indigestion and can be used to help those suffering from depression and anxiety. Recently it has been shown to have promising sleep-promoting effects, which may prove helpful for those who have insomnia and other sleep disorders | [General Description]
D-Threonine is sweet and toxic in nature.It acts as an inhibitor of L-threonine dehydratase. |
|
|