| Identification | More | [Name]
Captopril | [CAS]
62571-86-2 | [Synonyms]
(2S)-1-(3-MERCAPTO-2-METHYLPROPIONYL)-L-PROLINE
(2S)-N-[3-MARCAPTO-2-METHYLPROPIONYL]-L-PROLINE
(2S)-N-(3-MERCAPTO-2-METHYLPROPIONYL)-L-PROLINE
CAPTOPRIL
N-[(S)-3-MERCAPTO-2-METHYLPROPIONYL]-L-PROLINE
(S)-(-)-1-(3-MERCAPTO-2-METHYL-1-OXOPROPYL)-L-PROLINE
(S)-(-)-1-(3-MERCAPTO-2-METHYLPROPIONYL)-L-PROLINE
SQ-14225
(s,s)-1-(d-3-mercapto-2-methyl-1-oxopropyl)-l-proline
1-((2s)-3-mercapto-2-methylpropionyl)-l-proline
1-(3-mercapto-2-methyl-1-oxopropyl)-l-proline
1-(d-3-mercapto-2-methyl-1-oxopropyl)-l-proline(s,s)
1-(d-3-mercapto-2-methyl-1-propionyl)-,l-(s,s)-1-pyrrolidinecarboxylicaci
3-mercapto-2-methylpropionyl-proline
acediur
aceplus
acepress
acepril
alopresin
capoten | [EINECS(EC#)]
263-607-1 | [Molecular Formula]
C9H15NO3S | [MDL Number]
MFCD00168073 | [Molecular Weight]
217.29 | [MOL File]
62571-86-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
104-108 °C (lit.) | [alpha ]
-129.5 º (c=1, EtOH) | [Boiling point ]
427.0±40.0 °C(Predicted) | [density ]
1.2447 (rough estimate) | [refractive index ]
-127.5 ° (C=1.7, EtOH) | [storage temp. ]
room temp | [solubility ]
H2O: 0.1 g/mL, very slightly hazy, colorless
| [form ]
Crystalline Powder | [pka]
3.7, 9.8(at 25℃) | [color ]
white to off-white | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic (organic) | [Water Solubility ]
soluble | [Usage]
Orally active angiotensin-converting enzyme (ACE) inhibitor | [Merck ]
1774 | [BRN ]
477887 | [BCS Class]
3 | [CAS DataBase Reference]
62571-86-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Captopril(62571-86-2) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R43:May cause sensitization by skin contact.
R63:Possible risk of harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S22:Do not breathe dust . | [WGK Germany ]
2
| [RTECS ]
UY0550000
| [HS Code ]
29339900 | [Hazardous Substances Data]
62571-86-2(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 1040 i.v.; 6000 orally (Keim) |
| Hazard Information | Back Directory | [Description]
Captopril is the most studied of the angiotensin-converting enzyme inhibitors proposed as an
antihypertensive drug. It blocks angiotensin-converting enzyme, which suppresses formation
of angiotensin II and relieves its vasoconstricting effect on arterial and venous vessels. Overall
vascular peripheral tension is reduced, which results in the lowering of arterial pressure. | [Chemical Properties]
White or almost white, crystalline powder. | [Originator]
Lopirin,Von Heyden,W. Germany,1980 | [Uses]
anesthetic | [Uses]
angiotensin-converting enzyme (ACE) inhibitor,anti-hypertensive | [Uses]
Orally active angiotensin-converting enzyme (ACE) inhibitor | [Definition]
ChEBI: A L-proline derivative in which L-proline is substituted on nitrogen with a (2S)-2-methyl-3-sulfanylpropanoyl group. It is used as an anti-hypertensive ACE inhibitor drug. | [Manufacturing Process]
The first step is the manufacture of L-proline tert-butyl ester. L-proline (230 g)
is dissolved in a mixture of water (1 l) and 5 N sodium hydroxide (400 ml).
The solution is chilled in an ice bath, and under vigorous stirring, 5 N sodium
hydroxide (460 ml) and benzyloxycarbonyl chloride (340 ml) are added in five
equal aliquots during a half-hour period. After one hour stirring at room
temperature, the mixture is extracted twice with ether and acidified with
concentrated hydrochloric acid. The precipitate is filtered and dried. Yield is
442 g; MP 78°C to 80°C.
The benzyloxycarbonyl-L-proline thus obtained (180 g) is dissolved in a
mixture of dichloromethane (300 ml), liquid isobutylene (800 ml) and
concentrated sulfuric acid (7.2 ml). The solution is shaken in a pressure bottle
for 72 hours. The pressure is released, the isobutylene is allowed to evaporate
and the solution is washed with 5% sodium carbonate, water, dried over
magnesium sulfate and concentrated to dryness in vacuo, to obtain
benzyloxycarbonyl-L-proline tert-butyl ester, yield 205 g.
Benzyloxycarbonyl-L-proline tert-butyl ester (205 g) is dissolved in absolute
ethanol (1.2 l) and hydrogenated at normal pressure with 10% Pd on carbon
(10 g) until only a trace of carbon dioxide is observed in the hydrogen exit
gas (24 hours). The catalyst is filtered off and the filtrate is concentrated in
vacuo at 30 mm Hg. The residue is distilled in vacuo, to obtain L-proline tert-butyl ester, BP1mm 50°C to 51°C.
The next step yields 1-(3-acetylthio-2-methylpropanoyl)-L-proline tert-butyl
ester. L-proline tert-butyl ester (5.1 g) is dissolved in dichloromethane (40 ml)
and the solution stirred and chilled in an ice bath. Dicyclohexylcarbodiimide
(15 ml) is added followed immediately by a solution of 3-acetylthio-2-
methylpropanoic acid (4.9 g) in dichloromethane (5 ml). After 15 minutes
stirring in the ice bath and 16 hours at room temperature, the precipitate is
filtered off and the filtrate is concentrated to dryness in vacuo. The residue is
dissolved in ethyl acetate and washed neutral. The organic phase is dried over magnesium sulfate and concentrated to dryness in vacuo. The residue 1-(3-
acetylthio-2-methylpropanoyl)-L-proline tert-butyl ester is purified by column
chromatography (silica gel-chloroform), yield 7.9 g.
Then, 1-(3-acetylthio-2-methylpropanoyl)-L-proline is produced. The 1-(3-
acetylthio-3-methylpropanoyl)-L-proline tert-butyl ester (7.8 g) is dissolved in
a mixture of anisole (55 ml) and trifluoroacetic acid (110 ml). After one hour
storage at room temperature the solvent is removed in vacuo and the residue
is precipitated several times from ether-hexane. The residue (6.8 g) is
dissolved in acetonitrile (40 ml) and dicyclohexylamine (4.5 ml) is added. The
crystalline salt is boiled with fresh acetonitrile (100 ml), chilled to room
temperature and filtered, yield 3.8 g, MP 187°C to 188°C. This material is
recrystallized from isopropanol [α]D-67° (C 1.4, EtOH). The crystalline
dicyclohexylamine salt is suspended in a mixture of 5% aqueous potassium
bisulfate and ethyl acetate. The organic phase is washed with water and
concentrated to dryness. The residue is crystallized from ethyl acetate-hexane
to yield the 1-(3-acetylthio-2-D-methylpropanoyl)-L-proline, MP 83°C to 85°C.
Finally, Captopril is produced. The thioester (0.85 g) is dissolved in 5.5 N
methanolic ammonia and the solution is kept at room temperature for 2
hours. The solvent is removed in vacuo and the residue is dissolved in water,
applied to an ion exchange column on the H+ Cycle (Dowex 50, analytical
grade) and eluted with water. The fractions that give positive thiol reaction are
pooled and freeze dried. The residue is crystallized from ethyl acetate-hexane,
yield 0.3 g. The 1-(3-mercapto-2-D-methylpropanoyl)-L-proline has a melting
point of 103°C to 104°C. | [Brand name]
Capoten (Par). | [Therapeutic Function]
Antihypertensive | [Biological Functions]
Captopril (Capoten) is an orally effective ACE inhibitor
with a sulfhydryl moiety that is used in binding to the
active site of the enzyme. Captopril blocks the blood
pressure responses caused by the administration of angiotensin
I and decreases plasma and tissue levels of angiotensin
II. | [General Description]
Captopril, 1-[(2S)-3-mercapto-2-methyl-1-oxopropionyl]proline (Capoten), blocks the conversion of angiotensinI to angiotensin II by inhibiting the convertingenzyme. The rational development of captopril as an inhibitorof ACE was based on the hypothesis that ACE and carboxypeptidaseA functioned by similar mechanisms. It wasnoted that d-2-benzylsuccinic acid was a potent inhibitor ofcarboxypeptidase A, but not ACE. By use of this small molecule as a prototype, captopril was designed with a carboxylgroup on a proline and a thiol group was introduced toenhance the binding to the zinc ion of ACE. The importantbinding points at the active site of ACE are thought to be anarginine residue, which provides a cationic site that attracts acarboxylate ion, and a zinc ion, which can polarize a carbonylgroup of an amide function to make it more susceptible to hydrolysis.Hydrophobic pockets lie between these groups in theactive site, as does a functional group that forms a hydrogenbond with an amide carbonyl. | [Biochem/physiol Actions]
Angiotensin converting enzyme inhibitor. Inhibits the formation of angiotensin II, a bioactive peptide that stimulates angiogenesis and increases microvessel density. | [Pharmacology]
Treatment with captopril reduces blood pressure in
patients with renovascular disease and in patients with
essential hypertension.The decrease in arterial pressure
is related to a reduction in total peripheral resistance.
Most studies demonstrate a good correlation between
the hypotensive effect of inhibitors and the degree of
blockade of the renin–angiotensin system.Many of the
pharmacological effects of captopril are attributable to
the inhibition of angiotensin II synthesis. However,
ACE is a relatively nonselective enzyme that also catabolizes
a family of kinins to inactive products. Bradykinin, one of the major kinins, acts as a vasodilator
through mechanisms related to the production
of nitric oxide and prostacyclin by the vascular endothelium.
Thus, administration of the ACE inhibitor
captopril not only inhibits angiotensin II production but
also prevents the breakdown of bradykinin. Increases in
bradykinin concentrations after administration of ACE
inhibitors contribute to the therapeutic efficacy of these
compounds in the treatment of hypertension and congestive
heart failure. However, alterations in bradykinin concentrations are also thought to contribute to cough
and angioedema sometimes seen after ACE inhibition.
The hypotensive response to captopril is accompanied
by a fall in plasma aldosterone and angiotensin II
levels and an increase in plasma renin activity. Serum
potassium levels are not affected unless potassium supplements
or potassium-sparing diuretics are used concomitantly;
this can result in severe hyperkalemia.
There is no baroreflex-associated increase in heart rate,
cardiac output, or myocardial contractility in response
to the decrease in pressure, presumably because captopril
decreases the sensitivity of the baroreceptor reflex.
Captopril enhances cardiac output in patients with
congestive heart failure by inducing a reduction in ventricular
afterload and preload. Converting enzyme inhibitors
have been shown to decrease the mass and wall thickness of the left ventricle in both normal and hypertrophied
myocardium. ACE inhibitors lack metabolic
side effects and do not alter serum lipids. | [Clinical Use]
Captopril, as well as other ACE inhibitors, is indicated
in the treatment of hypertension, congestive heart
failure, left ventricular dysfunction after a myocardial
infarction, and diabetic nephropathy. In the treatment
of essential hypertension, captopril is considered firstchoice
therapy, either alone or in combination with a
thiazide diuretic. Decreases in blood pressure are primarily
attributed to decreased total peripheral resistance
or afterload. An advantage of combining captopril
therapy with a conventional thiazide diuretic is that the
thiazide-induced hypokalemia is minimized in the presence
of ACE inhibition, since there is a marked decrease
in angiotensin II–induced aldosterone release.
If the patient is asymptomatic, captopril can be used
as monotherapy in the treatment of congestive heart
failure. The use of ACE inhibitors in the treatment of
congestive heart failure is supported by results from
large-scale clinical trials demonstrating a general reduction
in the relative risk of death. In symptomatic patients
captopril should be used in conjunction with a diuretic
because of the weak natriuretic properties of
ACE inhibitors. In combination, captopril will reduce
afterload and preload and prevent diuretic-induced activation
of the renin–angiotensin system. Finally, ACE
inhibitors may slow the progression of congestive heart
failure by limiting left ventricular hypertrophy.
In the treatment of diabetic nephropathy associated
with type I insulin-dependent diabetes mellitus, captopril
decreases the rate of progression of renal insufficiency
and retards the worsening of renal function. | [Side effects]
Approximately 10% of the patients treated with
captopril report a dose-related maculopapular rash
that often disappears when the dosage of captopril is
reduced. Other common adverse effects are fever, a
persistent dry cough (incidence as high as 39%), initial
dose hypotension, and a loss of taste that may result in
anorexia. These effects are reversed when drug therapy
is discontinued. More serious toxicities include a
1% incidence of proteinuria and glomerulonephritis;
less common are leukopenia and agranulocytosis.
Since food reduces the bioavailability of captopril by
30 to 40%, administration of the drug an hour before
meals is recommended. All converting enzyme inhibitors
are contraindicated in patients with bilateral
renal artery disease or with unilateral renal artery disease
and one kidney. Use under these circumstances
may result in renal failure or paradoxical malignant
hypertension. | [Synthesis]
Captopril, 1-[(2S)-3-mercapto-2-methylpropionyl]-L-proline (22.7.4), is synthesized
by direct acylation of L-proline with 3-acetylthio-2-methylpropionic acid chloride
(22.7.2), which is synthesized from 3-acetylthio-2-methylpropionic acid (22.7.1), which is
in turn synthesized by reacting methacrylic and thioacetic acid. 1-(3-Acetylthio-2-Dmethylpropanoyl)-
L-proline (22.7.3) is formed by reacting L-proline with 3-acetylthio-2-
methylpropionic acid chloride, and it undergoes further ammonolysis with ammonia, to
give the desired captopril (22.7.4).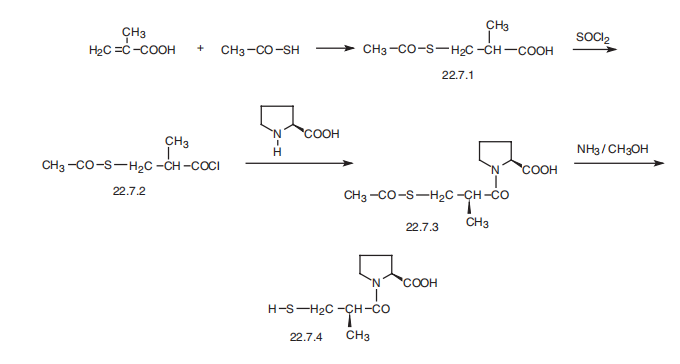
| [Veterinary Drugs and Treatments]
The principle uses of captopril in veterinary medicine, at present,
are as a vasodilator
in the treatment of CHF and in the treatment
of hypertension. Because of fewer adverse effects,
enalapril and
benazepril have largely supplanted the use of this drug in veterinary
medicine. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia,
hypotension and renal failure with ARBs and
aliskiren.
Bee venom extract: possible severe anaphylactoid
reactions when used together.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Cytotoxics: increased risk of angioedema with
everolimus.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Gold: flushing and hypotension with sodium
aurothiomalate.
Lithium: reduced excretion, possibility of enhanced
lithium toxicity.
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity | [Metabolism]
The onset of action following oral administration of
captopril is about 15 minutes, with peak blood levels
achieved in 30 to 60 minutes. Its apparent biological
half-life is approximately 2 hours, with its antihypertensive
effects observed for 6 to 10 hours. The kidneys appear
to play a major role in the inactivation of captopril. | [storage]
+4°C | [Purification Methods]
Purify it by recrystallisation from EtOAc/hexane. It is also purified by dissolving in EtOAc and chromatographed on a column of Wakogel C200 using a linear gradient of MeOH in EtOAc (0-100o) and fractions which give a positive nitroprusside test (for SH), are combined, evaporated and recrystallised from EtOAc/hexane (1:1), to give white crystals with [�] D -128.2o (c 2.0, EtOH). [Nam J Pharm Sci 73 1843 1984]. Alternatively, dissolve it in H2O, apply to a column of AG-50Wx2 (BioRad) and elute with H2O. The free acid is converted to the dicyclohexylamine salt in MeCN by addition of the amine until the pH is 8-9. The salt is converted to the free acid by shaking with EtOAc and 10% aqueous KHSO4 or passage through an AG50Wx2 column. The EtOAc solution is dried (MgSO4), evaporated to dryness and the residue is recrystallised as above from EtOAc/hexane [Cushman et al. Biochemistry 16 5484 1977, NMR and IR: Horii & Watanabe Yakugaku Zasshi (J Pharm Soc Japan) 81 1786 1961]. It is an antihypertensive because it is a potent competitive inhibitor of the angiotensive convertive enzyme (ACE-inhibitor) with a Ki value of 0.0017\M [Shimazaki et al. Chem Pharm Bull Jpn 30 3139 1982]. | [References]
1) Cushman?et al. (1999),?Design of angiotensin converting enzyme inhibitors; Nat.Med.,?5?1110
2) Orning?et al. (1991),?Inhibition of leukotriene A4 hydrolase/aminopeptidase by captopril; J.Biol.Chem.,?266?16507 |
|
|