| Identification | More | [Name]
Busulfan | [CAS]
55-98-1 | [Synonyms]
1�,4-Bis(methyl sulfonoxy)butane
1,4-BUTANEDIOL DIMETHANESULFONATE
1,4-Butanediol dimethyl sulfonate
1,4-dimethanesulfonoxybutane
BUSULFAN
busulfex
busulphan
myleran
tetramethylene bis(methanesulfonate)
(1,4-bis(methanesulfonyloxy)butane)
1,4-bis(methanesulfonoxy)butane
1,4-Bis(methane-sulponyloxy)butane
1,4-butanedioldimethanesulphonate
1,4-dimesyloxybutane
1,4-dimethanesulfonoxylbutane
1,4-dimethanesulfonyloxybutane
1,4-dimethanesulphonyloxybutane
1,4-dimethylsulfonoxybutane
1,4-dimethylsulfonyloxybutane
2041c.b. | [EINECS(EC#)]
200-250-2 | [Molecular Formula]
C6H14O6S2 | [MDL Number]
MFCD00007562 | [Molecular Weight]
246.3 | [MOL File]
55-98-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
114-117 °C(lit.) | [Boiling point ]
359.3°C (rough estimate) | [density ]
1.305 (estimate) | [refractive index ]
1.5630 (estimate) | [Fp ]
9℃ | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Very slightly soluble in water, freely soluble in acetone and in acetonitrile, very slightly soluble in ethanol (96 per cent). | [form ]
Crystalline Powder | [color ]
Pale Brown | [Water Solubility ]
Decomposes | [Usage]
Alkylating agent with antileukemic activity. Antineoplastic | [Merck ]
1505 | [BRN ]
1791786 | [Stability:]
Moisture Sensitive | [CAS DataBase Reference]
55-98-1(CAS DataBase Reference) | [IARC]
1 (Vol. 4, Sup 7, 100A) 2012 | [EPA Substance Registry System]
55-98-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,T | [Risk Statements ]
R45:May cause cancer.
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R63:Possible risk of harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 1
| [WGK Germany ]
3
| [RTECS ]
EK1750000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29053990 | [Safety Profile]
Confirmed carcinogen
producing leukemia, kidney, and uterine
tumors. Experimental neoplastigenic and
tumorigenic data. Poison by ingestion,
subcutaneous, intraperitoneal, intravenous,
and possibly other routes. Ingestion by
pregnant women can cause cancer of the
reproductive system of the fetus includtng
the uterus. Human teratogenic effects by
ingestion and possibly other routes include
developmental abnormaltties of the eye, ear,
craniofacial area including the nose and
tongue, gastrointestinal system, endocrine
system, urogenital system, and other
unspecified areas. Other human
reproductive effects by ingestion and
possibly other routes include: impotence,
changes in the uterus, cervix, and vagina,
and menstrual-cycle dtsorders. Experimental
reproductive effects. Human systemic
effects by ingestion: general arteriolar or
venous ddation of the eye, changes in
structure or function of salivary glands.
When heated to decomposition it emits
toxic fumes of SOx. See also
SULFONATES. | [Hazardous Substances Data]
55-98-1(Hazardous Substances Data) | [Toxicity]
LD50 i.v. in rats: 1.8 mg/kg (Scherf) |
| Hazard Information | Back Directory | [General Description]
White crystals or powder. | [Reactivity Profile]
MYLERAN(55-98-1) is an alkylating agent which hydrolyzes in water. . Strong reducers may yield hydrogen sulfide. | [Air & Water Reactions]
This compound is an alkylating agent which hydrolyzes in water. . | [Hazard]
Extremely toxic, carcinogen, clastogenic,
teratogenic, immunosuppressive, delayed bone
marrow aplasia, cataracts, pigmentation, pulmonary
thrombosis, cardiotoxic effects, thrombocytopenia. | [Fire Hazard]
Flash point data for this chemical are not available. MYLERAN is probably combustible. | [Description]
Chemically, busulfan is classified as an alkyl sulfonate. One or both of the methylsulfonate ester moieties can be displaced by the nucleophilic N7 of guanine, leading to monoalkylated and cross-linked DNA. The extent of alkyl sulfonate–mediated DNA interstrand cross-linking has been shown to vary with the length of the alkyl chain between sulfonate esters, with the tetramethylene-containing busulfan showing less interstrand cross-linking capability than hexamethylene, methylene, or octamethylene analogues. Intrastrand cross-linking also occurs, preferentially at 5′-GA-3′ but also at 5′-GG-3′ sequences. Alkylation of Cys sulfhydryl groups is yet another mechanism of cytotoxicity. | [Chemical Properties]
White Crystalline Solid | [Originator]
Myleran,Burroughs-
Wellcome,US,1954 | [Uses]
Alkylating agent with antileukemic activity. Antineoplastic | [Uses]
Antineoplastic alkylating agent, the palliative
treatment of chronic myeloid leukemia, and insect
sterilant.
| [Uses]
Busulfan USP (Myleran) is used to treat Chronic granulocytic leukemia; other myeloproliferative disorders. | [Definition]
ChEBI: A methanesulfonate ester that is butane-1,4-diol in which the hydrogens of the hydroxy groups are replaced by methanesulfonyl groups. An alkylating antineoplastic agent, it is used for the treatment of chronic myeloid leukemia (although it has been largely
replaced by newer drugs). It is also used as an insect sterilant. | [Indications]
Busulfan (Myleran) is a bifunctional methanesulfonic
ester that forms intrastrand cross-linkages with DNA.
The drug is well absorbed after oral administration and
has a plasma half-life of less than 5 minutes. Metabolites
and degradation products are excreted primarily in the
urine.
Busulfan is used in the palliative treatment of
chronic granulocytic leukemia. Daily oral therapy results
in decreased peripheral white blood cells and improved
symptoms in almost all patients during the
chronic phase of the disease. Excessive uric acid production
from rapid tumor cell lysis should be prevented
by coadministration of allopurinol.
At usual therapeutic dosages, busulfan is selectively
toxic to granulocyte precursors rather than lymphocytes.
Thrombocytopenia and anemia and less commonly,
nausea, alopecia, mucositis, and sterility also may
occur. Unusual side effects of busulfan include gynecomastia,
a general increase in skin pigmentation, and interstitial
pulmonary fibrosis. | [Manufacturing Process]
3.6 grams of redistilled 1,4-butanediol were dissolved in 10 ml of pyridine and
the solution was cooled in ice and water. 9.6 grams of redistilled methanesulfonyl-
chloride were added dropwise at such a rate that the temperature did
not rise above 20°C. The solution was then allowed to stand at room
temperature to; 30 minutes, during which time the temperature rose to 60°C.
A thick precipitate of pyridine hydrochloride was formed.
The mass was cooled in ice water and was treated with 30 ml of ice cold
water. On agitation, a white crystalline precipitate was formed. This was
filtered off and washed well with ice cold water and allowed to drain on the
pump. It weighed 7.8 grams and had a melting point of 100°C. 3.5 grams of
the material were recrystallized from acetone and ether to give small white
needles, having a melting point of 106°-107°C, unchanged by further
recrystallization. | [Brand name]
Myleran (GlaxoSmithKline). | [Therapeutic Function]
Antineoplastic | [Clinical Use]
Busulfan is used in the treatment of chronic myelogenous leukemia and can be administered either orally or by IV infusion.
| [Side effects]
Serious bone marrow hypoplasia and myelosuppression are possible with this agent, and recovery from busulfan�induced pancytopenia can take up to 2 years. | [Synthesis]
Busulfan, 1,4-butandioldimethansulfonate (30.2.3.1), is made by reacting
butandiol with methanesulfonyl chloride.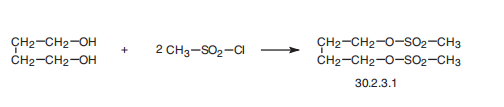
| [Potential Exposure]
Those involved in the manufacture,formulation, or use of this compound which finds application as an insect sterilant, and as a chemotherapeutic agenttaken orally to treat some kinds of leukemia. | [Veterinary Drugs and Treatments]
Busulfan may be useful in the adjunctive therapy of chronic granulocytic
leukemias or polycythemia vera in small animals. Not commonly
used in veterinary medicine. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
metronidazole.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Antifungals: metabolism inhibited by itraconazole,
monitor for signs of busulfan toxicity. | [First aid]
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately with wateror normal saline for 20� 30 min, occasionally lifting upperand lower lids. Seek medical attention immediately. If thischemical contacts the skin, remove contaminated clothingand wash immediately with soap and water. Seek medicalattention immediately. If this chemical has been inhaled,remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped.Transfer promptly to a medical facility. When this chemicalhas been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | [Carcinogenicity]
1,4-Butanediol dimethanesulfonate is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in
humans. | [Metabolism]
Busulfan is extensively metabolised in the liver, mainly
by conjugation with glutathione, either spontaneously or
mediated by the enzyme glutathione-S-transferase. About
12 inactive metabolites have been identified, which are
excreted in the urine. About 1% of busulfan is excreted
unchanged. Elimination in the faeces is considered to be
negligible. | [storage]
Store at -20°C | [Shipping]
The label requirement for medicine, solid, toxic,n.o.s. is “POISONOUS/TOXIC MATERIALS.” Medicine,solid, toxic, n.o.s., fall in Hazard Class 6.1 and busulfanfalls in Packing Group II. | [Incompatibilities]
Oxidizers, moist air, and water. | [References]
[1]. probin v, wang y, zhou d. busulfan-induced senescence is dependent on ros production upstream of the mapk pathway. free radic biol med, 2007, 42(12): 1858-1865.
[2]. probin v, wang y, bai a, et al. busulfan selectively induces cellular senescence but not apoptosis in wi38 fibroblasts via a p53-independent but extracellular signal-regulated kinase-p38 mitogen-activated protein kinase-dependent mechanism. j pharmacol exp ther, 2006, 319(2): 551-560.
[3]. choi yj, ok dw, kwon dn, et al. murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a fas/fasl- and p53-independent manner. febs lett, 2004, 575(1-3): 41-51. |
|
|