| Identification | More | [Name]
Mitotan | [CAS]
53-19-0 | [Synonyms]
1,1-dichloro-2,2-bis(2,4'-dichlorophenyl)ethane
1,1-DICHLORO-2-(2-CHLOROPHENYL)-2-(4-CHLOROPHENYL)ETHANE
1,1-DICHLORO-2-(O-CHLOROPHENYL)-2-(P-CHLOROPHENYL)ETHANE
1-(2-CHLOROPHENYL)-1-(4-CHLOROPHENYL)-2,2-DICHLOROETHANE
1-chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)benzene
2,2-(2-chlorophenyl)-2-(4-chlorophenyl)-1,1-dichloroethane
2-(2-CHLOROPHENYL)-2-(4-CHLOROPHENYL)-1,1-DICHLOROETHANE
2,4'-DDD
(2,4'-DICHLORODIPHENYL)DICHLOROETHANE
2,4'-TDE
CB-313
'LGC' (1126)
LYSODREN
MITOTAN
MITOTANE
O,P'-DDD
O,P'-TDE
1-(o-Chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane
1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-ethan
1,1-Dichloro-2-(p-chlorophenyl)-2-(o-chlorophenyl)ethane | [EINECS(EC#)]
200-166-6 | [Molecular Formula]
C14H10Cl4 | [MDL Number]
MFCD00000850 | [Molecular Weight]
320.04 | [MOL File]
53-19-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
77-78 °C(lit.)
| [Boiling point ]
405.59°C (rough estimate) | [density ]
1.3118 (rough estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
APPROX 4°C
| [solubility ]
DMSO: soluble20mg/mL, clear | [form ]
powder | [color ]
white to beige | [Water Solubility ]
<0.1 g/100 mL at 24 ºC | [Usage]
An Antineoplastic. Used as an adrenolytic agent | [Merck ]
13,6237/13,6237 | [BRN ]
2056007 | [InChI]
InChI=1S/C14H10Cl4/c15-10-7-5-9(6-8-10)13(14(17)18)11-3-1-2-4-12(11)16/h1-8,13-14H | [InChIKey]
JWBOIMRXGHLCPP-UHFFFAOYSA-N | [SMILES]
C1(Cl)=CC=CC=C1C(C1=CC=C(Cl)C=C1)C(Cl)Cl | [CAS DataBase Reference]
53-19-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Mitotane(53-19-0) | [EPA Substance Registry System]
53-19-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
KH7880000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2903990002 | [Hazardous Substances Data]
53-19-0(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
Colorless powder. | [Reactivity Profile]
O,P'-DDD(53-19-0) dehydrohalogenates with strong alkalis. Simple aromatic halogenated organic compounds are very unreactive; halogenated aliphatic compounds are moderately or very reactive. For both subgroups, reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. O,P'-DDD is probably combustible. | [Description]
Mitotane is an inhibitor of steroidogenesis that suppresses the growth of adrenocortical cells.1,2 It blocks the expression of several genes that encode proteins involved in steroidogenesis and disrupts mitochondrial respiratory chain activity in human adrenocortical cells.1,2 Mitotane has anti-neoplastic actions, alone or in combination with other compounds, and suppresses cortisol synthesis, although it also has significant toxicity in the gastrointestinal tract and nervous system.3,4 It is effective against adrenocortical carcinoma and Cushing’s Syndrome in clinical trials.4,5 | [Definition]
ChEBI: Mitotane is a diarylmethane. | [Indications]
Mitotane (Lysodren) produces selective atrophy of the
zona fasciculata and zona reticularis, which results in a
decrease in the secretion of 17-hydroxycorticosteroids.
Direct inhibition of cholesterol side-chain cleavage and
11�/18-hydroxylase activities has also been demonstrated. | [Indications]
The observation that mitotane (Lysodren) could produce
adrenocortical necrosis in animals led to its use in
the palliation of inoperable adrenocortical adenocarcinomas.
A reduction in both tumor size and adrenocortical
hormone secretion can be achieved in about half of
the patients taking the drug. Because normal adrenocortical
cells also are affected, endogenous glucocorticoid
production should be monitored and replacement
therapy administered when appropriate.
Mitotane is incompletely absorbed from the gastrointestinal
tract after oral administration. However,
once absorbed, it tends to accumulate in adipose tissue.
Mitotane is slowly excreted and will appear in the urine
for several years.The major toxicities associated with its
use are anorexia, nausea, diarrhea, lethargy, somnolence,
dizziness, and dermatitis. | [Manufacturing Process]
From dichloroacetaldehyde and 2-chlorphenylmagnesiumbromide was
prepared 1-(2-chlorphenyl-2,2-dichloroethanol. By action of H2SO4 on 1-(2-
chlorphenyl)-2,2-dichloroethanol in chlorobenzene was prepared 1,1-dichloro-
2,2-bis(2,4'-dichlorophenyl)ethane. | [Brand name]
Lysodren (Bristol-Myers Squibb). | [Therapeutic Function]
Antineoplastic | [Pharmacology]
Mitotane, a derivative of the insecticide DDT, quickly lowers the level of corticosteroids,
and is metabolized in the blood and urine and used on non-operable metastatic prostate
carcinomas. Synonyms of this drug are lysodren and others. | [Clinical Use]
Mitotane is the drug of choice for the treatment of
primary adrenal carcinoma when surgery or radiation
therapy is not feasible. Its effectiveness
in curtailing adrenal activity is due to an action on
adrenocortical mitochondria to impair cytochrome
P450 steps in steroid biosynthesis. Mitotane requires
metabolic transformation to exert its therapeutic action,
and the differential ability of tumors to metabolize
the drug may determine its clinical effectiveness. It is
advised to measure serum mitotane levels and urinary
free cortisol excretion to ensure adequate therapeutic
concentrations. Mitotane increases circulating cholesterol
by inhibiting cytochrome P450–mediated reactions
and therefore contributes to the cardiovascular
events that are a significant cause of mortality in untreated
Cushing’s syndrome.
Mitotane, being closely related to the organochlorine
insecticides, shares its inductive effects on the liver
microsomal drug-metabolizing enzyme system, and its
use may therefore alter the requirement for concomitantly
administered drugs that are also metabolized by
this pathway. | [Side effects]
Mitotane is capable of inducing remission of
Cushing’s disease, but only after several weeks of therapy
and at the price of severe gastrointestinal distress.
Moreover, more than half of patients relapse following
cessation of therapy. Other side effects include lethargy,
mental confusion, skin rashes, and altered hepatic function.
Being a lipid-soluble substance, mitotane remains
stored in body tissues for extended periods. This may
account for the marked patient-to-patient variability in
its therapeutic and/or toxic effects. | [Synthesis]
Mitotane, 1,1-dichloro-2-(o-chlorophenyl)ethane (30.5.8), is made by alkylating
chlorobenzene with 1-(2-chlorophenyl)-2,2-dichloroethane (30.5.7) in the presence of sul�furic acid. The necessary 1-(2-chlorophenyl)-2,2-dichloroethanol (30.5.7) is in turn made
from reacting 2-chlorophenylmagnesiumbromide with dichloroacetic aldehyde.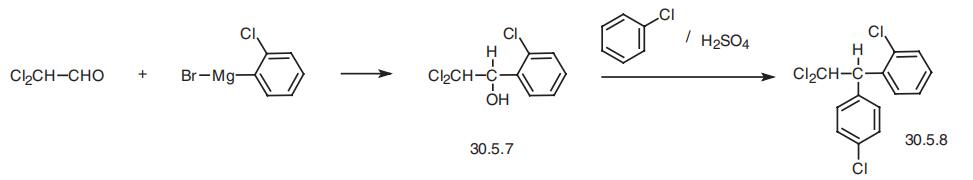
| [Veterinary Drugs and Treatments]
In veterinary medicine, mitotane is used primarily for the medical
treatment of pituitary-dependent hyperadrenocorticism (PDH),
principally in the dog. It has also been used for the palliative treatment
of adrenal carcinoma in humans and dogs. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: possibly reduced anticoagulant effect
of coumarins.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Diuretics: avoid with spironolactone. | [Metabolism]
Metabolised in the liver and other tissues and excreted as
metabolites in urine and bile. From 10-25% of a dose has
been recovered in the urine as a water-soluble metabolite
and 1-17% in the faeces as metabolites | [storage]
Store at -20°C | [Purification Methods]
Purify Mitotane by recrystallisation from pentane, MeOH or EtOH. It is soluble in isooctane and CCl4. [Haller et al. J Am Chem Soc 67 1600 1945, Beilstein 5 IV 1883.] |
| Questions And Answer | Back Directory | [Pharmacological effects]
Mitotan is structurally similar with insecticide DDT and DDD and can selectively cause the atrophy and necrosis of adrenal cortex-zona fasciculata and reticularis cells but without affecting the zona, therefore the secretion of aldosterone will not affected. After the drug administration, the cortisol and its metabolites level in blood and urine decreased rapidly after treatment. At the same time, the in vivo adrenocorticotropic hormone and metabolite products level also decrease rapidly. It is suitable for the treatment of inoperable, functional and non-functional adrenal cortical carcinoma, adrenal tumor, adrenal hyperplasia-caused Klinefelter’s syndrome, adrenal hyperplasia, and the adjuvant therapy of postoperation cortical cancer and tumor-induced Cushing's syndrome.
After oral administration, about 40% of Mitotan is absorbed through the gastrointestinal tract with the remaining 60% of the prototype excreted together with the feces. At a dose of 5~10 g daily, the plasma concentration can be up to 10~90μg/ml, the concentration of metabolites can be up to 30~50μg/ml. At 6-9 weeks after discontinuation, it can be still detected of o-alkyl chloride in the plasma. Mitotan has a high fat-solubility and is mainly stored in fat. The water soluble metabolites discharged from the urine can account for about 25% of the administered dose.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
It appears as white crystals with the melting point being 76-78 ℃. It is soluble in ethanol and carbon tetrachloride.
| [Uses]
It belongs to antineoplastic agents and can be used for the treatment of adrenal cortical carcinoma.
| [Production method]
It can be prepared from O-bromo-chlorobenzene (see 05820) by the following steps.
| [Category]
Toxic substances.
| [Toxicity grading]
Poisoning.
| [Acute toxicity]
Oral-rat; LD50> 5000 mg/kg; Oral-Mouse LD50> 4000 mg/kg.
| [Flammability and hazard properties]
Thermal decomposition can release toxic chloride fume.
| [Storage characteristics]
Low-temperature, dry and ventilated warehouse.
| [Extinguishing media]
Water, carbon dioxide, foam, powder. |
|
|