| Identification | More | [Name]
Atropic acid | [CAS]
492-38-6 | [Synonyms]
2-PHENYLACRYLIC ACID
2-PHENYLPROP-2-ENOIC ACID
2-Phenylpropenoic acid
ALPHA-PHENYLACRYLIC ACID
ATROPIC ACID
2-Propenoic acid, 2-phenyl-
Acrylic acid, 2-phenyl-
alpha-Toluic acid, alpha-methylene-
Benzeneacetic acide, alpha-methylene-
Propenoic acid, 2-phenyl-
alpha-Phenylacrylic acid
2-Phenylacrylic acid
2-Phenylprop-2-enoic acid
α-methylene-α-toluic acid
ATROPIC ACID/2-PHENYLACRYLIC ACID
10029 ATROPIC ACID
α-Methylenebenzeneacetic acid | [EINECS(EC#)]
207-753-6 | [Molecular Formula]
C9H8O2 | [MDL Number]
MFCD00046531 | [Molecular Weight]
148.16 | [MOL File]
492-38-6.mol |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [HS Code ]
29163990 |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
Atropic acid is an Impurity of Ipratropium bromide (I740500) and Atropine Sulfate (A794625). | [Preparation]
The synthesis of atropic acid involves several steps. Firstly, tropic acid is oxidized with hot KMnO4 to form benzoic acid. Secondly, tropic acid reacted with HBr to produce C9H9O2Br. Thirdly, C9H9O2Br is reacted with alcoholic KOH to yield atropic acid, C9H8O2. Finally, atropic acid is catalytically hydrogenated to form hydratropic acid, C9H10O2. | [Reactions]
Atropic acid(492-38-6) is formed by the dehydration of tropic acid. Hence addition of water to atropic acid gives tropic acid.
Atropic acid, on oxidation yields benzoic acid. The formation of benzoic acid reveals that atropic acid and tropic acid contain atleast one benzene nucleus with a side chain containing carboxylic acid in their structure.
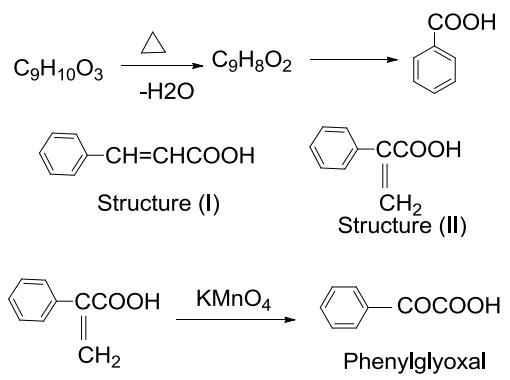
As atropic acid is an unsaturated acid it mean atropic acid may be either structure (I) or (II) .
Hence, the structure (II) is atropic acid which is confirmed by oxidation with KMnO4 to form phenylglyoxal. |
|
|