| Identification | More | [Name]
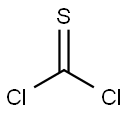
Thiophosgene | [CAS]
463-71-8 | [Synonyms]
THIOCARBONYL CHLORIDE
THIOPHOSGENE
Carbon chlorosulfide
carbonchlorosulfide
Carbonic dichloride, thio-
carbonothioicdichloride
Carbonyl chloride, thio-
CSCl2
Dichlorothiocarbonyl
Phosgene, thio-
Thiocarbonic dichloride
thio-carbonicdichlorid
thiocarbonicdichloride
Thiocarbonyl dichloride
thio-carbonylchlorid
thiocarbonyldichloride
Thiofosgen
thiofosgen(czech)
Thiokarbonylchlorid
thiokarbonylchlorid(czech) | [EINECS(EC#)]
207-341-6 | [Molecular Formula]
CCl2S | [MDL Number]
MFCD00004918 | [Molecular Weight]
114.98 | [MOL File]
463-71-8.mol |
| Chemical Properties | Back Directory | [Appearance]
A clear dark red to reddish-yellow liquid. Sharp, choking odor. | [Melting point ]
<25 °C | [Boiling point ]
73 °C | [density ]
1.508 | [vapor density ]
4 (vs air)
| [refractive index ]
n20/D 1.548(lit.)
| [Fp ]
62 °C
| [storage temp. ]
2-8°C
| [solubility ]
Chloroform | [form ]
Liquid | [color ]
A reddish liquid | [Stability:]
Stable. but reacts violently with water to produce toxic fumes. Incompatible with water, alcohols. Refrigerate at 2-8 C. | [Water Solubility ]
slow decomposition in cold, fast in hot water | [BRN ]
1633495 | [CAS DataBase Reference]
463-71-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Carbonothioic dichloride(463-71-8) | [EPA Substance Registry System]
463-71-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R22:Harmful if swallowed.
R23:Toxic by inhalation.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S7:Keep container tightly closed .
S9:Keep container in a well-ventilated place .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S7/9:Keep container tightly closed and in a well-ventilated place . | [RIDADR ]
UN 2474 6.1/PG 2
| [WGK Germany ]
1
| [RTECS ]
XN2450000
| [F ]
19-21 | [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
28530090 | [Safety Profile]
Poison by intravenous
route. Moderately toxic by ingestion. A skin,
mucous membrane, and severe eye irritant.
When heated to decomposition it emits very
toxic fumes of Cland SOx. See also
PHOSGENE. | [Hazardous Substances Data]
463-71-8(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Potassium iodide-->Sulfur dioxide-->1,1,2,2-Tetrachloroethane-->Perchloromethylmercaptan | [Preparation Products]
Fluorescein isothiocyanate isomer I-->5-ISOTHIOCYANATO-1,3-DIMETHYL-1H-PYRAZOLE-->2-(1,3-DIMETHYL-1H-PYRAZOL-5-YL)ETHENETHIONE-->3-ISOTHIOCYANATO-1,5-DIMETHYL-1H-PYRAZOLE-->2-ISOTHIOCYANATO-4,6-DIMETHOXYPYRIMIDINE-->5-BROMO-6-ISOTHIOCYANATE QUINOXALINE-->3,4-METHYLENEDIOXYPHENYL ISOTHIOCYANATE-->Amoscanate-->4-(1H-PYRAZOL-1-YL)PHENYL ISOTHIOCYANATE-->1,3-diisopropyl-2-isothiocyanato-5-phenoxybenzene-->1,4-PHENYLENE DIISOTHIOCYANATE-->DI-2-PYRIDYL THIONOCARBONATE-->4-CARBOXYPHENYL ISOTHIOCYANATE-->3-CHLORO-2-METHYLPHENYL ISOTHIOCYANATE-->2-ETHOXYPHENYL ISOTHIOCYANATE-->2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL ISOTHIOCYANATE-->3,5-DIMETHYLPHENYL ISOTHIOCYANATE-->2-CHLORO-4-METHYLPHENYL ISOTHIOCYANATE-->4-CHLORO-2-METHYLPHENYL ISOTHIOCYANATE-->3,5-DIMETHOXYPHENYL ISOTHIOCYANATE |
| Hazard Information | Back Directory | [General Description]
A reddish liquid. Boiling point 73.5°C. A severe eye irritant. May severely burn skin on contact. Very toxic by inhalation and by skin absorption. | [Reactivity Profile]
THIOPHOSGENE(463-71-8) is incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Liberates hydrogen sulfide upon reaction with acids. | [Air & Water Reactions]
Reacts with water to evolve hydrogen chloride, carbon disulfide, and carbon dioxide. Reaction is slow unless the water is hot. | [Health Hazard]
Inhalation causes irritation of respiratory system and delayed pulmonary edema. Vapor irritates eyes. Liquid burns skin and eyes. Ingestion causes irritation of mouth and stomach. | [Potential Exposure]
Primary irritant (w/o allergic reaction). There is not large-scale production of the chemical in the United States It is used to make other chemicals and in laboratory synthesis. | [First aid]
Get medical attention at once after any exposure to this compound. Inhalation: remove victim from exposure. Administer oxygen as soon as possible; support respiration; watch for pulmonary edema. Eyes: irrigate with large quantities of water for 15 minutes. Skin: flush with water. Ingestion: Do NOT induce vomiting; give large amount of water. | [Shipping]
UN2474 Thiophosgene, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard; Inhalation Hazard Zone B. PG 2. STN: 49 232 98. | [Incompatibilities]
Vapors may form explosive mixture with air. Incompatible with water and alcohols. Reacts with water releasing toxic hydrogen chloride, carbon disulfide, and carbon dioxide. Reaction is slow unless the water is hot. Decomposes above 200℃ to highly flammable carbon bisulfide and carbon tetrachloride. Corrodes metals, rubber and some plastics in the presence of moisture. Thiophosgene is incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Liberates hydrogen sulfide upon reaction with acids | [Chemical Properties]
A clear dark red to reddish-yellow liquid. Sharp, choking odor. | [Chemical Properties]
Reddish liquid. Decomposes in water and alcohol; soluble
in ether. | [Waste Disposal]
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. | [Uses]
Thiophosgene is a photo degradation product of the agricultural fungicide Folpet (F402000). | [Definition]
ChEBI: Thiophosgene is a thiocarbonyl compound and a one-carbon compound. | [Hazard]
Toxic by ingestion and inhalation. |
|
|