| Identification | More | [Name]
Tetrabutylammonium tribromide | [CAS]
38932-80-8 | [Synonyms]
TBABR3
TETRABUTYLAMMONIUM BROMIDE PERBROMIDE
TETRABUTYLAMMONIUM TRIBROMIDE
TETRA-N-BUTYLAMMONIUM TRIBROMIDE
1-Butanaminium,N,N,N-tributyl-,(tribromide)
Tetrabutylammonium perbromide
Tetra-n-butylammonium tribromide, 98+%
Tetra-n-butylammonium Tribromide(TBAB)
TETRA-N-BUTYLAMMONUIM TRIBROMIDE
TETRABUTYLAMMONIUM BROMIDE PERBROMIDE (TRIBROMIDE)
TBABr3, Tetrabutylammonium bromide perbromide
Tetrabutylammonium tribromide ,98% | [EINECS(EC#)]
609-598-3 | [Molecular Formula]
C16H36Br3N-2 | [MDL Number]
MFCD00012110 | [Molecular Weight]
482.18 | [MOL File]
38932-80-8.mol |
| Chemical Properties | Back Directory | [Appearance]
orange crystalline powder | [Melting point ]
71-76 °C (lit.) | [density ]
1.5469 (rough estimate) | [refractive index ]
1.6500 (estimate) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Acetone (Slightly), Chloroform (Slightly) | [form ]
Crystalline Powder | [color ]
Yellow | [Water Solubility ]
insoluble | [Detection Methods]
NMR | [BRN ]
3746114 | [InChIKey]
KEWPQWXPEDFWRU-UHFFFAOYSA-N | [CAS DataBase Reference]
38932-80-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
UN3261 | [WGK Germany ]
3
| [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29211990 |
| Questions and Answers (Q&A) | Back Directory | [Uses]
- Tetrabutylammonium tribromide (TBATB) is a salt of the lipophilic tetrabutylammonium cation and the linear tribromide anion. It is used as a phase transfer catalyst in organic synthesis as well as a mild brominating agent. A wide range of O-isopropylidene derivatives can be prepared from the sugars and their derivatives on reaction with acetone at room temperature by employing 2 mol % of tetrabutylammonium tribromide as a catalyst. Good yields, low catalyst loading, mild reaction conditions, and a non-aqueous workup procedure are some major advantages of this protocol.
- Tetrabutylammonium tribromide as an efficient generator of HBr for an efficient chemoselective reagent for acetalization of carbonyl compounds.
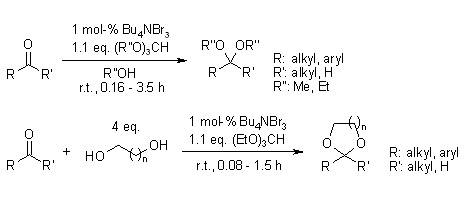
- It is sometimes used as a reagent used in organic synthesis as a conveniently weighable, solid source of bromine.
- Tetra-n-butylammonium tribromide is useful for the preparation of vicinal dibromides from alkenes and alkynes and alfa-bromo acetals.
- it is utilized to generate hydrogen bromide, which is used as an efficient chemoselective reagent for acetalization of carbonyl compounds.
|
|
|