| Identification | More | [Name]
Sulindac | [CAS]
38194-50-2 | [Synonyms]
(1Z)-5-FLUORO-2-METHYL-1-[[4-(METHYLSULFINYL)PHENYL]METHYLENE]-1H-INDENE-3-ACETIC ACID
5-FLUORO-2-METHYL-1Z-[[4(METHYLSILFUNYL)PHENYL]METHYLENE]-1H-INDENE-3-ACETIC ACID
AFLODAC
AKOS NCG1-0019
ALGOCETIL
LABOTEST-BB LT00772301
MK-231
SULINDAC
SULINOL
SULREUMA
(Z)-5-FLUORO-2-METHYL-1-[P-(METHYLSULFINYL)BENZYLIDENE]INDENE-3-ACETIC ACID
(z)-5-fluoro-2-methyl-1-((p-(methylsulfinyl)phenyl)methylene)-1h-indene-3-ac
1h-indene-3-aceticacid,5-fluoro-2-methyl-1-((4-(methylsulfinyl)phenyl)methyle
Aclin
arthrocine
cis-5-fluoro-2-methyl-1-((4-(methylsulfinyl)phenyl)methylene)-1h-indene-3-ac
cis-sulindac
clinoril
Clusinol
mobilin | [EINECS(EC#)]
253-819-2 | [Molecular Formula]
C20H17FO3S | [MDL Number]
MFCD00599589 | [Molecular Weight]
356.41 | [MOL File]
38194-50-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R63:Possible risk of harm to the unborn child.
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
- | [RIDADR ]
3249 | [WGK Germany ]
3 | [RTECS ]
NK8226000 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29309090 |
| Hazard Information | Back Directory | [Description]
Many non-steroidal anti-inflammatory drugs (NSAIDs) are potent but non-selective inhibitors of both COX-1 and COX-2 in humans.1 Sulindac is one of the older NSAIDs, an isostere of indomethacin developed before the inducible form of COX-2 was discovered.2 Although a number of NSAIDs have been found to protect against digestive tract cancers, sulindac has an extensive epidemiology documenting reduced human colorectal cancer. In murine models, sulindac was found not only to inhibit the enzymatic activity of polyp-associated COX-2, but also to downregulate the expression of colonic COX-2 protein to control levels.3 | [Chemical Properties]
Yellow Crystalline Solid | [Originator]
Imbaral,Sharp and Dohme ,W. Germany,1976 | [Uses]
A non-steroidal anti-inflammatory agent. An anti-inflammatory | [Uses]
Sulindac is a non-steroidal anti-inflammatory drug. | [Uses]
Sulindac is used for relieving weak to moderate pain in rheumatoid arthritis and
osteoarthritis. | [Definition]
ChEBI: A monocarboxylic acid that is 1-benzylidene-1H-indene which is substituted at positions 2, 3, and 5 by methyl, carboxymethyl, and fluorine respectively, and in which the phenyl group of the benzylidene moiety is substituted at the
ara position by a methylsulfinyl group. It is a prodrug for the corresponding sulfide, a non-steroidal anti-inflammatory drug, used particularly in the treatment of acute and chronic inflammatory conditions. | [Indications]
Sulindac (Clinoril) is chemically related to indomethacin
and is generally used for the same indications.
It is a prodrug that is metabolized to an active sulfide
metabolite and an inactive metabolite. The most
frequently reported side effects are GI pain, nausea, diarrhea,
and constipation. The incidence of these effects
is lower than for indomethacin, presumably because
sulindac is a prodrug and thus the active metabolite is
not highly concentrated at the gastric mucosa. As with
indomethacin, a rather high incidence of CNS side effects
(dizziness, headache) also occurs. | [Manufacturing Process]
The following process sequence is described in US Patent 3,654,349:
p-Fluoro-α-Methylcinnamic Acid: 200 grams (1.61 mols) pfluorobenzaldehyde,
3.5 grams (2.42 mols) propionic anhydride and 155
grams (1.61 mols) sodium propionate are mixed in a 1 liter three-necked flask
which had been flushed with nitrogen. The flask is heated gradually in an oilbath
to 140°C. After 20 hours the flask is cooled to 100°C and the contents
are poured into 8 liters of water. The precipitate is dissolved by adding 302
grams potassium hydroxide in 2 liters of water. The aqueous solution is
extracted with ether, and the ether extracts washed with potassium hydroxide
solution. The combined aqueous layers are filtered, acidified with concentrated
HCl, filtered and the collected solid washed with water, thereby producing pfluoro-
α-methylcinnamic acid which is used as obtained.p-Fluoro-α-Methylhydrocinnamic Acid: To 177.9 grams (0.987 mol) p-fluoro-α-
methylcinnamic acid in 3.6 liters ethanol is added 11.0 grams of 5% Pd/C and
the mixture reduced at room temperature under a hydrogen pressure of 40
psi. Uptake is 31/32 pounds (97% of theoretical). After filtering the catalyst,
the filtrate is concentrated in vacuo to give the product p-fluoro-α-
methylhydrocinnamic acid used without weighing in next step.
6-Fluoro-2-Methylindanone: To 932 grams polyphosphoric acid at 70°C on the
steam bath is added 93.2 grams (0.5 mol) p-fluoro-α-methylhydrocinnamic
acid slowly with stirring. This temperature is gradually raised to 95°C and the
mixture kept at this temperature for 1 hour. The mixture is allowed to cool
and added to 2 liters of water. The aqueous layer is extracted with ether, the
ether solution washed twice with saturated sodium chloride solution, 5%
Na2CO3 solution, water, and then dried. The ether filtrate is concentrated with
200 grams silica-gel, and added to a five pound silica-gel column packed with
5% ether-petroleum ether. The column is eluted with 5 to 10% etherpetroleum
ether and followed by TLC to give 6-fluoro-2-methylindanone.
5-Fluoro-2-Methylindene-3-Acetic Acid: A mixture of 18.4 grams (0.112 mol)
of 6-fluoro2-methylindanone, 10.5 grams (0.123 mol) cyanacetic acid, 6.6
grams acetic acid and 1.7 grams ammonium acetate in 15.5 ml dry toluene is
refluxed with stirring for 21 hours, as the liberated water is collected in a
Dean Stark trap. The toluene is concentrated and the residue dissolved in 60
ml of hot ethanol and 14 ml of 2.2 N aqueous potassium hydroxide solution.
22 grams of 85% KOH in 150 ml of water is added and the mixture refluxed
for 13 hours under N2. The ethanol is removed under vacuum, 500 ml water
added, the aqueous solution washed well with ether and then boiled with
charcoal. The aqueous filtrate is acidified to pH 2 with 50% hydrochloric acid,
cooled and the precipitate collected in this way dried 5-fluoro-2-methylindenyl-
3-acetic acid (MP 164° to 166°C) is obtained.
5-Fluoro-2-Methyl-1-(p-Methylthiobenzylidene)-3-Indenylacetic Acid: 15 grams
(0.072 mol) 5-fluoro-2-methyl-3-indenylacetic acid, 14.0 grams (0.091 mol)
p-methylthiobenzaldehyde and 13.0 grams (0.24 mol) sodium methoxide are
heated in 200 ml methanol at 60°C under nitrogen with stirring for 6 hours.
After cooling the reaction mixture is poured into 750 milliliters of ice-water,
acidified with 2.5 N hydrochloric acid and the collected solid triturated with a
little ether to produce 5-fluoro-2-methyl-1-(p-methylthiobenzylidene)-3-
indenylacetic acid (MP 187° to 188.2°C).
5-Fluoro-2-Methyl-1-(p-Methylsulfinylbenzylidene)-3-Indenylacetic Acid: To a
solution of 3.4 grams (0.01 mol) 5-fluoro-2-methyl-1-(pmethylthiobenzylidene)-
3-indenylacetic acid in a 250 ml mixture of methanol
and 100 ml acetone is added a solution of 3.8 grams (0.018 mol) of sodium
periodate in 50 ml water with stirring.
450 ml water is added after 18 hours and the organic solvents removed under
vacuum below 30°C. The precipitated product is filtered, dried and
recrystallized from ethyl acetate to give 5-fluoro-2-methyl-1-(pmethylsulfinylbenzylidene)-
3-indenylacetic acid. Upon repeated
recrystallization from ethylacetate there is obtained cis-5-fluoro-2-methyl-1-
(p-methylsulfinylbenzylidene)-3-indenylacetic acid (MP 184° to 186°C). | [Brand name]
Clinoril (Merck). | [Therapeutic Function]
Antiinflammatory | [General Description]
Sulindac, (Z)-5-fluoro-2-methyl-1-([p-(methylsulfinyl)phenyl]methylene)-1H-indene-3-acetic acid (Clinoril), isan NSAID prodrug that contains a chiral sulfoxide moietybut is marketed as the racemate because it undergoes invivo reduction by the hepatic enzymes into its achiral, activemetabolite, methyl sulfide that exhibits potent andnonselective COX inhibition similar to indomethacin.
The parent sulfoxide has a plasma half-life of 8 hours, andthe active methyl sulfide metabolite is 16.4 hours. The morepolar and inactive sulfoxide is virtually the only form excretedinto the renal tubules, thus sulindac is believed to haveminimal nephrotoxicity associated with indomethacin. Thelong half-life of sulindac is caused by the extensive enterohepaticcirculation and reactivation of the inactive sulfoxideexcreted. Coadministration of aspirin is contraindicated becauseit considerably reduces the sulfide blood levels. Carefulmonitoring of patients with a history of ulcers is recommended.Gastric bleeding, nausea, diarrhea, dizziness, andother adverse effects have been noted with sulindac, but witha lower frequency than with aspirin. Sulindac is recommendedfor RA, OA, and ankylosing spondylitis. | [Biological Activity]
Prodrug. Metabolizes to sulindac sulfide, a cyclooxgenase inhibitor that represses ras signaling, and sulindac sulfone, an antitumor agent, following oral administration in vivo . Widely used anti-inflammatory agent. | [Biochem/physiol Actions]
Nonsteroidal anti-inflammatory; preferential inhibitor of COX-1. | [Mechanism of action]
Sulindac induces no relevant COXinhibition
whereas the active metabolite sulindac sulfide
inhibits both isoenzymes with some COX-1
preference , indicating that the pharmacological
activity of sulindac probably results from
its sulfide metabolite. Another metabolite, sulindac
sulfone, induces apoptosis in tumor cells and
sulindac is extensively studied for cancer treatment
. Sulindac is administered orally or
rectally (200–400 mg/d). | [Pharmacokinetics]
Sulindac is well absorbed on oral administration (90%), reaches peak plasma levels within 2 to 4 hours, and being
acidic (pKa = 4.5), is highly bound to serum proteins (93%). The metabolism of sulindac plays a major role in its
actions, because all of the pharmacological activity is associated with its major metabolite. Sulindac is, in fact, a
pro-drug, the sulfoxide function being reduced to the active sulfide metabolite. Sulindac is absorbed as the sulfoxide,
which is not an inhibitor of prostaglandin biosynthesis in the GI tract. Prostaglandins exert a
protective effect in the GI tract, and inhibition of their synthesis here leads to many of the GI side effects noted for
most NSAIDs. Once sulindac enters the circulatory system, it is reduced to the sulfide, which is an inhibitor of
prostaglandin biosynthesis in the joints. Thus, sulindac produces less GI side effects, such as bleeding, ulcerations,
and so on, than indomethacin and many other NSAIDs. In addition, the active metabolite has a plasma half-life
approximately twice that of the parent compound (~16 hours versus 8 hours), which favorably affects the dosing
schedule. In addition to the sulfide metabolite, sulindac is oxidized to the corresponding sulfone, which is inactive. A
minor product results from hydroxylation of the benzylidene function and the methyl group at the 2-position.
Glucuronides of several metabolites also are found. Sulindac as well as the sulfide and the sulfone metabolites are
all highly protein-bound. Despite the fact that the sulfide metabolite is a major activation product and is found in high
concentration in human plasma, it is not found in human urine, perhaps because of its high degree of protein binding. | [Clinical Use]
Sulindac is indicated for long-term use in the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis,
and acute gouty arthritis. The usual maximum dosage is 400 mg/day, with starting doses recommended at 150 mg
twice a day. It is recommended that sulindac be administered with food. | [Side effects]
Whereas the toxicity of sulindac is lower than that observed for indomethacin and other NSAIDs, the spectrum of
adverse reactions is very similar. The most frequent side effects reported are associated with irritation of the GI tract
(e.g., nausea, dyspepsia, and diarrhea), although these effects generally are mild. Effects on the CNS (e.g.,
dizziness and headache) are less common. Dermatological effects are less frequently encountered. | [Synthesis]
Sulindac, 5-fluoro-2-methyl-1-[n-(methylsulfinyl)benzyliden]inden-3-acetic acid
(3.2.67) is synthesized in a multi-step synthesis from n-fluorobenzaldehyde, which upon con�densation with propionic acid anhydride in the presence of sodium acetate gives 4-fluoro-|á-
methylcinnamic acid (3.2.62). Reduction of the double bond by hydrogene using a palladium
on carbon catalyst gives 4-fluoro-|á-methyldihydrocinnamic acid (3.2.63). In the presence of
polyphosphoric acid, the resulting product undergoes cyclization to 5-fluoro-2-methyl-3-
indanone (3.2.64). The resulting ketone undergoes a Knoevenagel reaction with cyanoacetic
acid and is further decarboxylated into 5-fluoro-2-methyliden-3-acetic acid (3.2.65).
Condensation of the product with n-mercaptobenzaldehyde in the presence of sodium
methoxide gives 5-fluoro- 2-methyl-1-(4-methylthiobenzyliden)-3-indenacetic acid (3.2.66),
and the sulfur atom is oxidized by sodium periodate into the desired sulfoxide (3.2.67),
sulindac [119¨C122].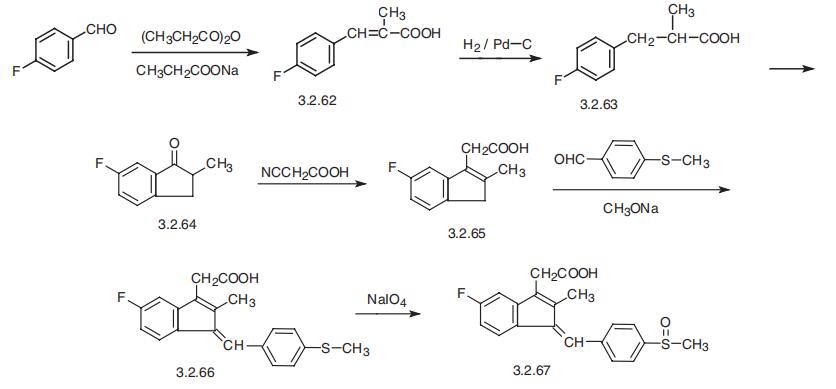
| [Drug interactions]
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Dimethyl sulfoxide: avoid concomitant use.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. | [Metabolism]
Sulindac is metabolised by reversible reduction to the
sulfide metabolite, which appears to be the active form,
and by irreversible oxidation to the sulfone metabolite.
About 50% is excreted in the urine mainly as the sulfone
metabolite and its glucuronide conjugate, with smaller
amounts of sulindac and its glucuronide conjugate; about
25% appears in the faeces, primarily as sulfone and sulfide
metabolites. Sulindac and its metabolites are also excreted
in bile and undergo extensive enterohepatic circulation. | [storage]
Store at -20°C |
|
|