| Identification | More | [Name]
Formamidine acetate | [CAS]
3473-63-0 | [Synonyms]
FAA
FORMAMIDINE ACETATE
FORMAMIDINE ACETATE SALT
FORMAMIDINE,ACETIC ACID
FORMAMIDINE ACETIC ACID SALT
IMINOFORMAMIDE ACETATE
Methanimidamideacetate
Formamidinium acetate
formamidine monoacetate
Formamidineacetate,99%
Formamidinmonoacetat
Methanimidamide monoacetate
Acetic acid·formamidine | [EINECS(EC#)]
222-442-5 | [Molecular Formula]
C3H8N2O2 | [MDL Number]
MFCD00012866 | [Molecular Weight]
104.11 | [MOL File]
3473-63-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystalline powder | [Melting point ]
158-161 °C (dec.)(lit.)
| [density ]
1.124 | [storage temp. ]
Store below +30°C. | [solubility ]
832g/l | [form ]
Liquid | [color ]
Clear colorless to light yellow | [PH]
8 (400g/l, H2O, 20℃) | [Water Solubility ]
832 g/L (21 ºC) | [Sensitive ]
Hygroscopic | [BRN ]
3563488 | [InChI]
InChI=1S/C2H4O2.CH4N2/c1-2(3)4;2-1-3/h1H3,(H,3,4);1H,(H3,2,3) | [InChIKey]
XPOLVIIHTDKJRY-UHFFFAOYSA-N | [SMILES]
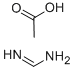
C(C)(=O)O.C(=N)N | [CAS DataBase Reference]
3473-63-0(CAS DataBase Reference) | [EPA Substance Registry System]
3473-63-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R43:May cause sensitization by skin contact.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S27:Take off immediately all contaminated clothing . | [WGK Germany ]
2
| [F ]
3-9-21 | [Autoignition Temperature]
565 °C | [TSCA ]
Yes | [HS Code ]
29252000 | [Toxicity]
LD50 orally in Rabbit: 2510 mg/kg LD50 dermal Rat > 2000 mg/kg |
| Hazard Information | Back Directory | [Description]
Formamidine acetate is an anti-tuberculosis drug that belongs to the class of aminoglycosides. It has been shown to have a potent cytotoxic effect on malignant brain cells in vitro. It inhibits bacterial growth by binding to DNA-dependent RNA polymerase, thereby preventing transcription and replication. The high frequency of human activity has been shown using a patch-clamp technique on human erythrocytes. This active form is metabolized through a number of metabolic transformations, including hydrolysis by esterases or glucuronidases, oxidation by cytochrome P450 enzymes, reduction by glutathione reductase, or conjugation with glucuronic acid. It also specifically binds to markers expressed at high levels in Mycobacterium tuberculosis strains (e.g., ESX-1 secretion system protein) and inhibits cell growth in culture. | [Chemical Properties]
white to light yellow crystalline powder | [Uses]
Formamidine acetate is used as an intermediate in the synthesis of active pharmaceutical ingredients. It is also used as a condensing agent for the preparation of pyrimidine and imidazole heterocycles. | [Purification Methods]
Unlike the hydrochloride, the acetate salt is not hygroscopic. It is recrystallised from a small volume of acetic acid, by addition of EtOH and the crystals are washed with EtOH then Et2O and dried in a vacuum. [Taylor et al. Org Synth 46 39 1966, Beilstein 2 IV 82.] |
|
|