| Identification | More | [Name]
Lithium triflate | [CAS]
33454-82-9 | [Synonyms]
LITHIUM TRIFLATE
LITHIUM TRIFLUOROMETHANESULFONATE
LITHIUM TRIFLUOROMETHANESULPHONATE
LITHIUM TRIFLUOROMETHYL SULFONATE
PFM-LI
TRIFLUOROMETHANESULFONIC ACID LITHIUM SALT
Methanesulfonicacid,trifluoro-,lithiumsalt
trifluoro-methanesulfonicacilithiumsalt
Lithium triflate~Trifluoromethanesulphonic acid lithium salt
LITHIUM TRIFLUOROMETHANESULFONATE, 99.99 5%
Lithium Trifluoro Methylsulphate
Lithiumtrifluoromethanesulfonate,99%(Lithiumtriflate)
Lithium trifluoromethanesulphonate 97%
Lithiumtrifluoromethanesulphonate97%
LITHIUM TRIFLUOROMETHANESULFONATE (LITHIUM TRIFLATE)
LITHIUM TRIFLUOROMETHENESULFONATE
Lithium trifluoromethanesulfonate, 98+%
Lithium triflate, Trifluoromethanesulfonic acid lithium salt | [EINECS(EC#)]
251-528-5 | [Molecular Formula]
CF3LiO3S | [MDL Number]
MFCD00013227 | [Molecular Weight]
156.01 | [MOL File]
33454-82-9.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [F ]
3-10 | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29049090 |
| Hazard Information | Back Directory | [Chemical Properties]
white powder | [Properties and Applications]
Lithium trifluoromethanesulfonate is highly soluble in water and organic solvents and shows excellent ion conductivity. It is highly soluble in polar organic solvents such as dimethyl carbonate (DMC), diethyl carbonate (DEC), ethyl acetate, and others. This compound exhibits good thermal stability and has a non-coordinating anion (triflate), which helps prevent unwanted reactions with other components in electrochemical systems.
| [Uses]
Lithium trifluoromethanesulfonate is mixed with ceramic filler to prepare polyethylene oxide films in order to improve the electrochemical and mechanical characteristics of the membrane, which is used in the lithium batteries. It acts as a doping salt used in the preparation of nano manganese-composite polymer electrolytes. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here. | [Synthesis]
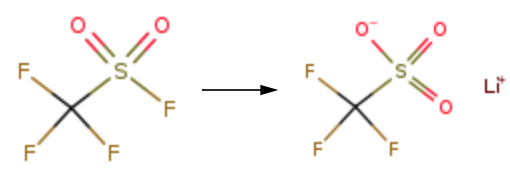
A 316L stainless steel autoclave was charged with 1678 g of a 10.0% aqueous solution of lithium hydroxide and 424 g of calcium oxide, and the temperature was raised to 85 ° C, and the mixture was stirred for 3 hours. The reaction pressure was controlled by continuously feeding the trifluoromethane sulfonyl fluoride gas. The reaction time is 3.5 hours, then the reaction is kept for 3 hours, and then the temperature is lowered to 25 ℃. The reaction liquid is filtered to obtain the product lithium trifluoromethanesulfonate aqueous solution.
|
|
|