| Identification | More | [Name]
7-Nitro-1,2,3,4-tetrahydroquinoline | [CAS]
30450-62-5 | [Synonyms]
1,2,3,4-TETRAHYDRO-7-NITROQUINOLINE
7-NITRO-1,2,3,4-TETRAHYDROQUINOLINE
7-NITRO-1,2,3,4-TETRAHYDRO-QUINOLINE HYDROCHLORIDE
7-Nitro-1,2,3,4-Terahydroquinoline | [EINECS(EC#)]
634-828-4 | [Molecular Formula]
C9H10N2O2 | [MDL Number]
MFCD00496654 | [Molecular Weight]
178.19 | [MOL File]
30450-62-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
61.0 to 65.0 °C | [Boiling point ]
325.2±31.0 °C(Predicted) | [density ]
1.236±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
soluble in Methanol | [form ]
Powder | [pka]
3.44±0.20(Predicted) | [color ]
Red | [CAS DataBase Reference]
30450-62-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
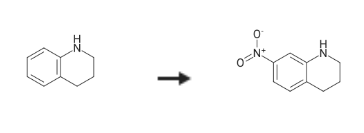
7-Nitro-1,2,3,4-tetrahydroquinoline is a quinoline compound that can be used as a reagent in chemical reactions or as an intermediate in organic synthesis. It can be used to prepare 7-Aminoquinoline and 7-Nitroquinoline. | [Synthesis]
7-Nitro-1,2,3,4-tetrahydroquinoline is synthesised by the reaction of 1,2,3,4-tetrahydroquinoline and nitric acid in sulphuric acid solution. The specific synthesis steps are as follows:
Concentrated sulfuric acid (30.00 mL) was cooled to -10° C. with an ice/salt bath.
To this, 1,2,3,4-tetrahydro-quinoline (10.60 g, 75.60 mmol) and a solution of nitric acid (99.5percent, 4.80 g, 75.60 mmol) in sulfuric acid (15.00 mL) were added simultaneously within 1 hour, so that the temperature of the reaction mixture does not exceed 10° C.
The mixture was stirred then for 2.5 hours at -5° C., then poured over ice and treated with sodium carbonate (0.10 kg) until pH 8-9 was reached.
The solid was filtered, washing with water, then dissolved in dichloromethane.
The organic phase was washed with water, dried over magnesium sulphate and evaporated.
7-Nitro-1,2,3,4-tetrahydro-quinoline was obtained as a viscous brown oil, 13.70 g (85percent), 84percent purity.
 |
|
|