| Identification | More | [Name]
2-(Trimethylsilyl)ethanol | [CAS]
2916-68-9 | [Synonyms]
2-HYDROXYETHYLTRIMETHYLSILANE
2-(TRIMETHYLSILYL)ETHANAL
2-(TRIMETHYLSILYL)ETHANOL
TIMTEC-BB SBB009030
2-(trimethylsilyl)-ethano
Ethanol, 2-(trimethylsilyl)-
Silane, (2-hydroxyethyl)trimethyl-
trimethylsilylethanol
(2-hydroxyethyl)trimethyl-silan
2-(TRIMETHYLSILYL)ETHANOL, 98+%
2-(Trimethylsilyl)ethyl alcohol | [EINECS(EC#)]
220-844-5 | [Molecular Formula]
C5H14OSi | [MDL Number]
MFCD00002825 | [Molecular Weight]
118.25 | [MOL File]
2916-68-9.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR COLOURLESS LIQUID | [Boiling point ]
71-73 °C35 mm Hg(lit.)
| [density ]
0.825 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.423(lit.)
| [Fp ]
123 °F
| [storage temp. ]
0-6°C | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Liquid | [pka]
15.38±0.10(Predicted) | [color ]
Clear colorless | [Specific Gravity]
0.825 | [Water Solubility ]
soluble | [Hydrolytic Sensitivity]
4: no reaction with water under neutral conditions | [BRN ]
1732034 | [Stability:]
Hygroscopic | [InChIKey]
ZNGINKJHQQQORD-UHFFFAOYSA-N | [CAS DataBase Reference]
2916-68-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Trimethyl-2-hydroxyethylsilane(2916-68-9) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,F | [Risk Statements ]
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1987 3/PG 3
| [WGK Germany ]
3
| [RTECS ]
KM5480000
| [Hazard Note ]
Flammable | [TSCA ]
No | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29310095 | [Toxicity]
mouse,LD50,intraperitoneal,1122mg/kg (1122mg/kg),Doklady Akademii Nauk SSSR. Proceedings of the Academy of Sciences of the USSR. For English translation, see DBIOAM and DKBSAS. Vol. 229, Pg. 1011, 1976. |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR COLOURLESS LIQUID | [Physical properties]
bp 50–52 °C/10 mmHg, 71–73 °C/35 mmHg;
d 0.825 g cm?3. | [Uses]
2-(Trimethylsilyl)ethanol is a protecting reagent for carboxyl, phosphoryl, hydroxyl, and amino
groups. It participates in the reactions of Phenol and Acid Protection, Alcohol Protection, Hemiacetal Protection, Amine Protection, Enol Ether Synthesis, Carbohydrate Chemistry etc. | [Preparation]
Three methods of preparation have been
reported: (a) from the treatment of ethyl bromoacetate with
zinc followed by the reaction with chlorotrimethylsilane1 and
subsequent reduction of the resultant ethyl trimethylsilylacetate
with lithium aluminum hydride2,3 or borane¨Ctetrahydrofuran(eq 1); (b) from the hydroboration/oxidation or oxymercuration/demercuration of vinyltrimethylsilane (eq 2); and (c)most conveniently, by the reaction of the Grignard reagent
formed from (chloromethyl)trimethylsilane with paraformaldehyde
(eq 3).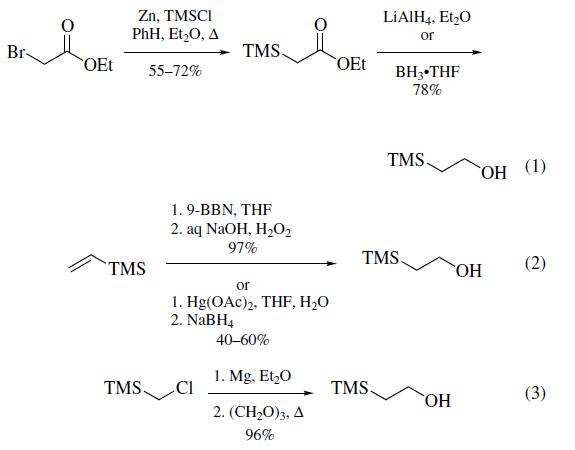
| [Purification Methods]
If the NMR spectrum is not clean, then dissolve the alcohol in Et2O, wash it with aqueous NH4Cl solution, dry (Na2SO4), evaporate and distil it. The 3,4-dinitrobenzoyl derivative has m 66o (from EtOH). [NMR: Speier et al. J Am Chem Soc 79 974 1957, Z Naturforsch 14b 137 1959, Beilstein 4 IV 3951.] |
|
|