| Identification | Back Directory | [Name]
Guanfacine | [CAS]
29110-47-2 | [Synonyms]
C07037
Guanfacine
Guanfacine USP/EP/BP
Guanfacine Solution, 100ppm
[(2,6-Dichlorophenyl)acetyl]guanidine
2-(2,6-Dichlorophenylacetyl)guanidine
1-(α-Oxo-2,6-dichlorophenethyl)guanidine
N-Amidino-2-[2,6-dichlorophenyl] acetamide
N-carbaMiMidoyl-2-(2,6-dichlorophenyl)acetaMide
BenzeneacetaMide, N-(aMinoiMinoMethyl)-2,6-dichloro-
N-(diaminomethylidene)-2-(2,6-dichlorophenyl)acetamide | [EINECS(EC#)]
249-442-8 | [Molecular Formula]
C9H9Cl2N3O | [MDL Number]
MFCD00865944 | [MOL File]
29110-47-2.mol | [Molecular Weight]
246.09 |
| Chemical Properties | Back Directory | [Melting point ]
227-230?C | [density ]
1.50±0.1 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
Soluble in DMSO | [form ]
Powder | [pka]
11.92±0.46(Predicted) | [BCS Class]
1 |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Estulic,Sandoz,Switz.,1980 | [Uses]
Centrally acting α-adrenoceptor agonist. Antihypertensive. | [Uses]
Guanfacine centrally acting α-adrenoceptor agonist. Guanfacine is an antihypertensive. | [Definition]
ChEBI: Guanfacine is a member of acetamides. | [Manufacturing Process]
2,6-Dichlorophenyl-acetyl-guanidine: A solution of 3.245 g (0.055 mol) of
guanidine in isopropanol is added to a solution of 11.7 g (0.05 mol) of 2,6-
dichlorophenyl-acetic acid ethyl ester (BP 142°C to 143°C/12 mm of Hg) in
20cc of isopropanol. The reaction mixture is allowed to stand overnight and is
subsequently concentrated by evaporation. After recrystallizing the residue
from methanol/ether 2,6-dichlorophenyl-acetyl-guanidine is obtained in the
form of white grains having a MP of 225°C to 227°C.
2,6-Dichlorophenyl-acetyl-guanidine hydrochloride: A solution of 5.6 g (0.025
mol) of 2,-dichlorophenylacetic acid chloride (BP 137°C to 138°C/12 mm of
Hg) in 10 cc of toluene is added dropwise to a mixture of 4.5 g (0.076 mol) of
guanidine and 60 cc of toluene. The reaction mixture is allowed to stand at
room temperature for 20 minutes, is then heated on a steam bath for 2 hours
and is subsequently cooled. The resulting precipitate is filtered off and washed
twice with 25 cc amounts of water in order to separate the guanidine
hydrochloride. The residue (2,6-dichlorophenyl-acetyl-guanidine) is washed
with chloroform for further purification and is then dissolved in 50 cc of
isopropanol. The pH-value of the solution is adjusted to 6 with ethanolic
hydrochloric acid and the solution is cooled. The resulting white needles are
again washed with chloroform. The resulting 2.6-dichlorophenyl-acetyl�guanidine hydrochloride has a MP of 213°C to 216°C. | [Brand name]
Tenex (Dr. Reddy’s). | [Therapeutic Function]
Antihypertensive | [General Description]
Guanfacine is more selective for 2-receptors than isclonidine. Their mechanism of action is the same as that ofclonidine. Differences between clonidine and its twoanalogs are seen in their elimination half-life values and intheir metabolism and urinary excretion patterns. The eliminationhalf-life of clonidine ranges from 20 to 25 hours,whereas that for guanfacine is about 17 hours. Guanabenzhas the shortest DOA of these three agents, with a half-lifeof about 6 hours. Clonidine and guanfacine are excretedunchanged in the urine to the extent of 60% and 50%,respectively. Very little of guanabenz is excreted unchangedin the urine. | [Synthesis]
Guanfacin, N-amidino-2-(2,6-dichlorophenyl)acetamide (22.3.2), is also synthesized
in a very easy synthesis of reacting the acid chloride or ester of 2,6-
dichlorophenylacetic acid with guanidine.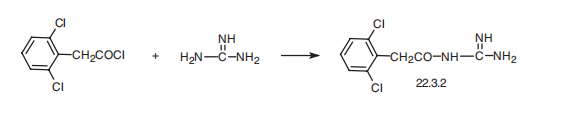
|
|
| Company Name: |
Harman Finochem Ltd
|
| Tel: |
+91-2226528080 +91-2226528080 |
| Website: |
www.harmanfinochem.com |
|