| Identification | More | [Name]
CLONIDINE (200 MG) | [CAS]
4205-90-7 | [Synonyms]
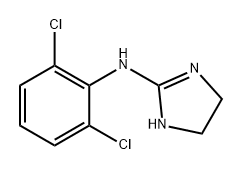
1H-Imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-
2-((2,6-dichlorophenyl)amino)-2-imidazoline
2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2)
2-(2,6-dichloroanilino)-2-imidazolin
2-(2,6-dichloroanilino)-2-imidazoline
2-(2,6-Dichlorophenylamino)-2-imidazoline
2,6-dichloro-n-2-imidazolidinylidene-benzenamin
2,6-dichloro-n-2-imidazolidinylidenebenzenamine
2-[(2,6-Dichlorophenyl)imino]imidazolidine
2-[(2,6-Dichlorophenyl)imino]imidazoline
2-Imidazoline, 2-(2,6-dichloroanilino)-
734571A
Benzenamine, 2,6-dichloro-N-2-imidazolidinylidene-
Catapres-TTS
Clonidin
m-5041t
n-(2,6-dichlorophenyl)-4,5-dihydro-1h-imidazol-2-amin
N-(2,6-Dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine
ST-155-BS
CLONIDINE (200 MG) | [EINECS(EC#)]
224-119-4 | [Molecular Formula]
C9H10Cl3N3 | [MDL Number]
MFCD00055059 | [Molecular Weight]
266.555 | [MOL File]
4205-90-7.mol |
| Hazard Information | Back Directory | [Uses]
Antihypertensive. | [Uses]
Clonidine
may be useful in the treatment of acute opioid withdrawal. Epidural
clonidine 1–2μgkg –1 increases the duration and potency of analgesia
provided by epidural opioid or local anaesthetic drugs. | [Definition]
ChEBI: A clonidine that is 4,5-dihydro-1H-imidazol-2-amine in which one of the amino hydrogens is replaced by a 2,6-dichlorophenyl group. | [Brand name]
Catapres (Boehringer
Ingelheim). | [Pharmacokinetics]
Clonidine is lipid soluble and rapidly absorbed after oral administration,
with a peak plasma concentration occurring in 60–90min. O ral, intravenous
and intramuscular routes may be used for sedation or analgesia. I n addition,
epidural and intrathecal clonidine is used to augment regional anaesthesia,
but perineural administration is of limited or no effect. The elimination halflife
is 9–13h and is prolonged in renal failure. Fifty percent of an
administered dose is excreted unchanged by the kidneys, and 50% is
metabolised in the liver to inactive metabolites. | [Clinical Use]
Clonidine is an α2-adrenergic
agonist and is primarily used in the cardiovascular
field for blood pressure reduction . The compound
induces analgesia via central α2-receptor
interaction and can be used orally, parenterally
or epidurally for pain treatment, often in
combination with opioids or local anesthetics
. Epidural clonidine – opioid combinations
are preferentially used for neuropathically maintained
cancer pain. The compound is additionally
used for migraine prophylaxis and to reduce
opioid and alcohol withdrawal symptoms .
Clonidine frequently induces side effects like
hypotension, sedation, drowsiness, dry mouth,
and constipation. The compound is well absorbed
orally. | [Physiological effects]
Clonidine has some effects at α1-receptors (α2/ α1 > 200 : 1).
Clonidine reduces the MA C of inhalational anaesthetic agents by up to 50%.
I t has a synergistic analgesic effect with opioids which may be partly
pharmacokinetic because the elimination half-life of opioids is also
increased. | [Physiological effects]
CNS effects
Clonidine produces sedation, anxiolysis and analgesia. I t also has a MA Csparing
effect, but there is a ceiling to the reduction because of the potential
for activity at α1 receptors when used at higher doses.
CVS effects
The cardiovascular effects of clonidine probably involve α1 receptors and
imidazoline receptors as with dexmedetomidine. Clonidine lowers the set
point around which arterial pressure is regulated.
Respiratory effects
Clonidine has minor respiratory effects, causing only a small reduction in
minute ventilation. | [Metabolic pathway]
Clonidine is well absorbed orally, with peak plasma
concentrations after 60–90min. I t is highly lipid soluble, and approximately
50% is metabolised in the liver to inactive metabolites; the rest is excreted
unchanged via the kidneys, with an elimination half-life of 9–12h. |
|
|