| Identification | More | [Name]
3-Bromobiphenyl | [CAS]
2113-57-7 | [Synonyms]
3-BROMOBIPHENYL
3-BROMODIPHENYL
BROMOBIPHENYL(3-)
PBB NO 2
1,1’-(biphenyl,3-bromo-
1,1’-biphenyl,3-bromo-(3-bromobiphenyl)
3-Bromo-1,1'-biphenyl
3-bromo-1’-biphenyl
3-bromo-bipheny
Biphenyl, 3-bromo-
m-Bromobiphenyl
3-BROMOBIPHNYL
pbb 2
3-BROMOBISPHENOLA
[1,1'-Biphenyl]-3-yl bromide
1-Bromo-3-phenylbenzene
3-Xenyl bromide
Biphenyl-3-yl bromide | [EINECS(EC#)]
218-304-9 | [Molecular Formula]
C12H9Br | [MDL Number]
MFCD00000082 | [Molecular Weight]
233.1 | [MOL File]
2113-57-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Colorless to pale yellow liquid | [Melting point ]
93-94 °C | [Boiling point ]
300 °C
| [density ]
1.398 g/mL at 25 °C(lit.) | [refractive index ]
1.637-1.641
| [Fp ]
110 °C
| [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Chloroform (Soluble), DMSO (Slightly) | [form ]
Powder | [color ]
Light yellow to gray-beige | [Water Solubility ]
Insoluble | [Merck ]
4188 | [BRN ]
2043191 | [InChIKey]
USYQKCQEVBFJRP-UHFFFAOYSA-N | [CAS DataBase Reference]
2113-57-7(CAS DataBase Reference) | [NIST Chemistry Reference]
| [EPA Substance Registry System]
2113-57-7(EPA Substance) |
| Questions And Answer | Back Directory | [Description]
3-Bromobiphenyl is an important industrial raw material, but also a very
widely used organic synthesis intermediates, it is widely used in
pharmaceutical, liquid crystal industry and other fields. In recent
years, the export volume of 3-Bromobiphenyl in our country is increasing
day by day, the main export object is some liquid crystal display
production enterprises in Japan and South Korea. | [synthesis]
Add 1-bromo-3-iodobenzene (4.67 g, 16.5mmol), phenylboronic acid (1.83 g, 15.0 mmol), Pd (OAc)2 (101 mg, 0.450 mmol), PPh3 (239 mg, 0.911 mmol), and K2CO3
(6.23 g, 45.1 mmol) to a 200 mL twonecked round-bottom flask. Add
toluene (40 mL) and water (40 mL) via syringe and reflux the mixture
(bath temp. 100°C) for 24 h. Extract the resulting mixture with ethyl
acetate (30 mL x 3). Dry the combined organic layer over Na2SO4 and concentrate in vacuo. Purify the residue to silica gel column chromatography (eluent:hexane).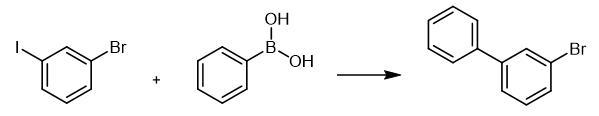 |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [RTECS ]
DV1750000 | [TSCA ]
Yes | [HS Code ]
29039990 | [Toxicity]
mouse,LDLo,intraperitoneal,500mg/kg (500mg/kg),"Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 6, Pg. 217, 1954. |
| Hazard Information | Back Directory | [General Description]
Clear yellow viscous liquid. Insoluble in water. | [Reactivity Profile]
3-BROMOBIPHENYL(2113-57-7) may be sensitive to light. This compound may react with oxidizing materials. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. 3-BROMOBIPHENYL is probably combustible. | [Chemical Properties]
Colorless to pale yellow liquid | [Uses]
3-Bromobiphenyl is utilized as an electrochemical immunosensor based on poly(dopamine) coated gold nanocluster for brominated flame retardants. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 41, p. 24, 1976 DOI: 10.1021/jo00863a005 | [Research]
3-Bromobiphenyl could be prepared by irradiation of 3,4-dibromobiphenyl (BpBr2) in acetonitrile. At present, a variety of detection methods have been developed to detect its concentration. Such as the electrochemical immunoassay of 3-BBP based on the
PDOP/PB/CMK-3 platform and the multi-HRP–DHCNTs–Ab2 signal
label. This detection method can detect the concentration of 3-Bromobiphenyl in the environment (This study takes river water from the estuary of the Pearl River as sample solvent)[1-2]. | [References]
[1] Sun Z, et al. Electrochemical immunosensor based on hydrophilic
polydopamine-coated prussian blue-mesoporous carbon
for the rapid screening of 3-bromobiphenyl. Biosensors and Bioelectronics, 2014; 59: 99-105.
[2] Peter K, et al.The photochemistry of polyhaloarenes XIII. The photohydrodehalogenation of 3,4-dibromobiphenyl. Tetrahedron, 1996; 52: 8397-8406. |
|
|