| Identification | Back Directory | [Name]
(S)-(+)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole,min.98%(S)-DTBM-SEGPHOS | [CAS]
210169-40-7 | [Synonyms]
(S)-DTBM-SEGPHOS
(S)-DTBM-SEGPHOS(R)
(S)-DTBM-SEGPHOS(R) >=94%
(S)-(+)-DTBM-SEGPHOS(regR)
(S)-5,5'-Bis[bis(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4'-bibenzodioxole
(S)-(+)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole
(S)-(+)-5,5μ-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4μ-bi-1,3-benzodioxole
(S)-(+)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole, min. 98%
Phosphine,[(4S)-[4,4'-bi-1,3-benzodioxole]-5,5'-diyl]bis[bis[3,5-bis(1,1-diMethylethyl)-4-Methoxyphenyl]-
(S)-(+)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole,min.98%(S)-DTBM-SEGPHOS
(S)-(+)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole, min. 98% (S)-(+)-DTBM-SEGPHOS(R) | [Molecular Formula]
C74H100O8P2 | [MDL Number]
MFCD09753003 | [MOL File]
210169-40-7.mol | [Molecular Weight]
1179.54 |
| Questions And Answer | Back Directory | [Reaction]
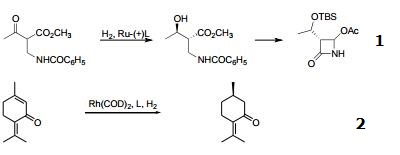
- Biaryl bisphosphine ligand with narrow dihedral angle. The DTBM SEGPHOS® ligand, as the ruthenium complex, gives superior enantioselectivity and diastereoselectivity through dynamic kinetic resolution in the asymmetric hydrogenation of a-substituted-β-ketoesters useful in the synthesis of carbapenum antibiotics.
- With rhodium, preferential enantioselective hydrogenation of more reactive olefin of extended enone structure.
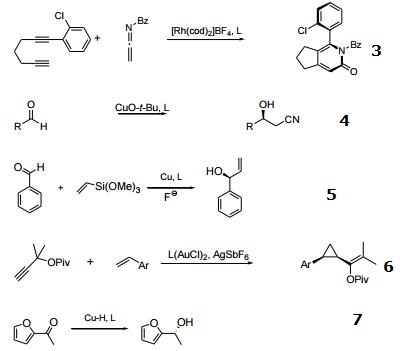
- Rhodium catalyzed chemo-, regio, and entantioselective [2 + 2 + 2] cycloaddition of alkynes with isocyanates.
- With copper, enantioselective cross Aldol-type reaction of acetonitrile.
- With copper, enantioselective vinylsilane alkenylation of aldehydes.
- Gold carbene mediated stereoselective cyclopropanation of propargyl esters.
- With copper, enantioselective 1,2-reduction of ketones, and 1,4-reduction of a α,β-usaturated esters.
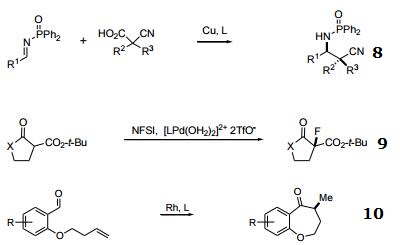
- With copper, catalytic enantioselective Mannich-type reaction.
- Enantioselective fluorination of b β-keto esters, tert-butoxycarbonyl lactones and lactmes with Sodeoka's Pd-aqua complex and a fluorinating reagent.
- Rh-catalyzed intramolecular olefin or carbonyl hydroacylation.
|
| Hazard Information | Back Directory | [Uses]
Catalytic ligand for:
- Enantioselective synthesis of secondary allylic alcohols via enantioselective reductions of α,β-unsaturated ketones
- Stereoselective preparation of cyclohexanone derivatives via rhodium-catalyzed rearrangement of allyl or allenic cyclobutanols
- Cu-catalyzed asymmetric conjugate reduction of beta-substituted unsaturated phosphonates to give optically active beta-stereogenic alkylphosphonates
- Asymmetric synthesis of cyclohexenones via Rh-catalyzed desymmetrization through enantioselective C-C bond activation and ring expansion of allenyl cyclobutanols
- Enantioselective synthesis and crystal structure of P-stereogenic alkynylphosphine oxides by Rh-catalyzed [2+2+2] cycloaddition
|
|
|