| Identification | More | [Name]
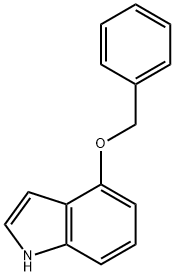
4-Benzyloxyindole | [CAS]
20289-26-3 | [Synonyms]
4-BENZYLOXY-1H-INDOLE
4-BENZYLOXYINDOLE
4-BENZYLOXYLINDOLE
4-(phenylmethoxy)-1h-indole
NSC 92539
4-BENZYLOXYINDOLE 98%
4-Benzyloxyindole ,98% | [EINECS(EC#)]
243-690-0 | [Molecular Formula]
C15H13NO | [MDL Number]
MFCD00047200 | [Molecular Weight]
223.27 | [MOL File]
20289-26-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Crystalline Solid with Few Darker (Brown) Particles | [Melting point ]
57-61 °C (lit.) | [Boiling point ]
364.56°C (rough estimate) | [density ]
1.0707 (rough estimate) | [refractive index ]
1.5500 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
17.17±0.30(Predicted) | [color ]
Beige to Brown | [Usage]
Indole derivative as substrate-binding; N297Q and I300V mutants of cytochrome P 450 2A6 display expansion of substrate specificity of cytochrome P 450 2A6 to oxidize substituted indoles | [Detection Methods]
HPLC,NMR | [BRN ]
183150 | [InChI]
InChI=1S/C15H13NO/c1-2-5-12(6-3-1)11-17-15-8-4-7-14-13(15)9-10-16-14/h1-10,16H,11H2 | [InChIKey]
LJFVSIDBFJPKLD-UHFFFAOYSA-N | [SMILES]
N1C2=C(C(OCC3=CC=CC=C3)=CC=C2)C=C1 | [CAS DataBase Reference]
20289-26-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29339900 |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Crystalline Solid with Few Darker (Brown) Particles | [Uses]
4-Benzyloxyindole was used in the synthesis of 4-alkyloxy-aminoalkyl indole derivatives. | [Uses]
Indole derivative as substrate-binding; N297Q and I300V mutants of cytochrome P 450 2A6 display expansion of substrate specificity of cytochrome P 450 2A6 to oxidize substituted indoles | [Synthesis Reference(s)]
Organic Syntheses, Coll. Vol. 7, p. 34, 1990
The Journal of Organic Chemistry, 51, p. 4294, 1986 DOI: 10.1021/jo00372a037
Tetrahedron Letters, 24, p. 4561, 1983 DOI: 10.1016/S0040-4039(00)85955-9 | [Synthesis]
To a stirred solution of 162.2 g (0.50 mol) of (E)-6-benzyloxy-2-nitro-β-pyrrolidinostyrene in 1 L of THF and 1 L of methanol at 30°C under nitrogen is added 10 mL of Raney nickel followed by 44 mL (0.75 mol) of 85% hydrazine hydrate. Vigorous gas evolution is observed. The red color turns dark brown within 10 minutes, and the reaction temperature rises to 46°C. An additional 44 mL of 85% hydrazine hydrate is added after 30 min and again 1 hr later. The temperature is maintained between 45 and 50°C with a water bath during the reaction and for 2 hr after the last addition. The mixture is cooled to room temperature, and the catalyst is removed by filtration through a bed of Celite. The catalyst is washed several times with methylene chloride. The filtrate is evaporated, and the residue is dried by evaporating with 500 mL of toluene. The reddish residue (118.5 g), dissolved in ca. 1 L of toluene-cyclohexane (1 : 1), is applied to a column of 500 g of silica gel (70–230-mesh, Merck) prepared in the same solvent. Elution with 6.0 L of toluene–cyclohexane (1 : 1) followed by 3 L of toluene–cyclohexane (1 : 2) affords 108.3 g of white solid, which is crystallized from 150 mL of toluene and 480 mL of cyclohexane. 107.3 g (96% yield) of 4-benzyloxyindole is obtained in three crops as white prisms, mp 60–62°C.
|
| Questions and Answers (Q&A) | Back Directory | [Reactions]
4-Benzyloxyindole was used in the synthesis of 4-alkyloxy-aminoalkyl indole derivatives.
- Reactant for regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition
- Reactant for preparation of indoles by Bartoli reductive cyclization as useful intermediates in medicinal chemistry research
- Reactant for synthesis of carbon-11-labeled 4-aryl-4H-chromenes as new PET agents for imaging of apoptosis in cancer
- Reactant for preparation of HCV inhibitors.
- Reactant for preparation of indol-3-yl tetramethylcyclopropyl ketones as CB2 cannabinoid receptor ligands
- Reactant for preparation of 4-aryl-4H-chromenes as apoptosis inducers
|
|
|