| Identification | More | [Name]
4-BROMO (1H)INDAZOLE | [CAS]
186407-74-9 | [Synonyms]
4-BROMO (1H)INDAZOLE
4-BROMOINDAZOLE | [EINECS(EC#)]
803-241-7 | [Molecular Formula]
C7H5BrN2 | [MDL Number]
MFCD05664001 | [Molecular Weight]
197.03 | [MOL File]
186407-74-9.mol |
| Chemical Properties | Back Directory | [Appearance]
brown powder | [Melting point ]
165-167° | [Boiling point ]
333.8±15.0 °C(Predicted) | [density ]
1.770±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
soluble in Ethanol | [form ]
Solid | [pka]
12.78±0.40(Predicted) | [color ]
Off-white | [InChI]
InChI=1S/C7H5BrN2/c8-6-2-1-3-7-5(6)4-9-10-7/h1-4H,(H,9,10) | [InChIKey]
KJIODOACRIRBPB-UHFFFAOYSA-N | [SMILES]
N1C2=C(C(Br)=CC=C2)C=N1 | [CAS DataBase Reference]
186407-74-9(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
4-Bromo-1H-indazole is a derivative indazole which has a heterocyclic structure made up of benzene and pyrazole rings. Indazoles are an important class of heterocyclic compounds with a wide range of application as biologic and pharmaceutic agents. Indazole derivatives have a wide range of biological activities. For instance, indazole derivatives show vasorelaxant and anti-aggregator activities by stimulating NO release and increasing cGMP levels. Recent medical research and development studies resulted in the production of indazole derivatives for the treatment of osteoporosis, inflammatory diseases and neurodegenerative disorders. 4-bromo-1H indazole molecule is a much stronger inhibitor of 6-bromo-1H indazole and 7-bromo-1H indazole. 4-Bromo-1H-indazole may be strong inhibitor candidate for LPO and that caution should be exercised when using medicines containing indazole derivatives[1].
| [Chemical Properties]
brown powder | [Synthesis]
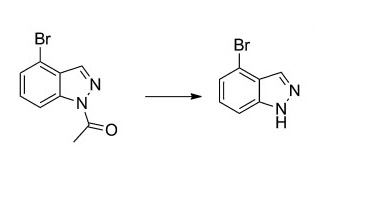
To N-Acetyl-4-bromo-1H-indazole (1.6 g, 6.69 mmol) was added methanol (25.9 mL) and then 6 N HCl (15.6 mL). The resulting solution was stirred at room temperature until the reaction was completed after 7 h (monitored by TLC, petroleum ether/ethyl acetate 2:1). After evaporation of methanol in vacuo, the residue was extracted with ethyl acetate (50 mL*3). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude was purified by flash chromatography (petroleum ether/ethyl acetate, 5:1) to yield 4-bromo-1H-indazole (0.8 g) as milky white solid, mp 164–166°C, yield 67.1%[2].
 | [References]
[1] Koksal Z, et al. Lactoperoxidase, an antimicrobial enzyme, is inhibited by some indazoles. Drug and Chemical Toxicology, 2018; 43: 22-26.
[2] Wang Y, et al. Synthesis and Antibacterial Activity of Novel 4-Bromo-1H-Indazole Derivatives as FtsZ Inhibitors. Archiv der Pharmazie, 2015; 348: 266-274. |
|
|