| Identification | Back Directory | [Name]
SELEGILINE | [CAS]
14611-51-9 | [Synonyms]
Emsam
C07245
Selgene
Zelapar
Selegina
Emsam TTS
SELEGELIN
SELEGILINE
dl-Depreny
l-Deprenyl
(-)-DEPRENYL
(-)-selegiline
SELEGILINE BASE
R(-)-SELEGILINE
(R)-(-)-Deprenyl
SELEGILINE USP/EP/BP
SELEGILINEHYDROCHLORDE
R(-)-Selegiline solution
Selegiline hydrochloride CRS
(RS)-Selegiline hydrochloride CRS
phenylisopropyl-n-MethylpropinylaMine
N-Methyl-N-[(R)-1-benzylethyl]-2-propyne-1-amine
(αR)-N,α-Dimethyl-N-(2-propynyl)benzeneethanamine
N-(Methyl-d3),a-methyl-N-2-propynyl Phenethylamine
(2R)-N-methyl-1-phenyl-N-prop-2-ynylpropan-2-amine
(R)-N,alpha-Dimethyl-N-2-propynylbenzeneethanamine
Benzeneethanamine, N,a-dimethyl-N-2-propynyl-, (R)-
BenzeneethanaMine, N,a-diMethyl-N-2-propyn-1-yl-, (aR)-
Benzeneethanamine, N,α-dimethyl-N-2-propyn-1-yl-, (αR)-
N-(Methyl-d3),a-methyl-N-2-propyn-1-yl-benzeneethanamine
Phenethylamine, N,a-dimethyl-N-2-propynyl-, L-(-)- (8CI)
Benzeneethanamine, N,a-dimethyl-N-2-propynyl-, (aR)- (9CI) | [EINECS(EC#)]
604-507-3 | [Molecular Formula]
C13H17N | [MDL Number]
MFCD00672171 | [MOL File]
14611-51-9.mol | [Molecular Weight]
187.28 |
| Chemical Properties | Back Directory | [Melting point ]
137.5-139 °C | [alpha ]
D20 -11.2° | [Boiling point ]
273℃ | [density ]
0.954 | [refractive index ]
nD20 1.5180 | [Fp ]
108℃ | [storage temp. ]
?20°C | [pka]
7.53±0.50(Predicted) | [InChI]
InChI=1/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/s3 | [InChIKey]
MEZLKOACVSPNER-PLAQIDKDNA-N | [SMILES]
C1(C=CC=CC=1)C[C@@H](C)N(C)CC#C |&1:7,r| | [CAS DataBase Reference]
14611-51-9 |
| Hazard Information | Back Directory | [Description]
Selegiline, a monoamine oxidase (MAO) inhibitor, is FDA-approved as an adjunct treatment in the management of patients with Parkinson disease and as a treatment for a major depressive disorder (MDD) in adults. Selegiline is also used off-label for early Parkinson disease and the treatment of attention-deficit/hyperactivity disorder (ADHD). | [Uses]
Antidyskinetic; antiparkinsonian (in combination with levodopa/carbidopa). | [Definition]
ChEBI:(-)-selegiline is a selegiline and a terminal acetylenic compound. It has a role as a geroprotector. It is a conjugate base of a (-)-selegiline(1+).
| [Brand name]
Emsam (Somerset). | [World Health Organization (WHO)]
Selegiline was introduced in the early 1990s. It is a monoamine
oxidase inhibitor and is used in the management of Parkinson's disease. A
symptomatic effect of selegiline in Parkinson's disease has been shown, but longer
follow-up failed to provide any definitive evidence of ability to retard the loss of
dopaminergic neurons (Parkinson's Study Group, 1993). | [Biological Functions]
Another drug used in the treatment of Parkinson’s disease
is selegiline (also known as deprenyl, or Eldepryl).
It is an irreversible inhibitor of MAO-B, an important
enzyme in the metabolism of dopamine (Fig. 33.2).
Blockade of dopamine metabolism makes more
dopamine available for stimulation of its receptors.
Selegiline, as monotherapy, may be effective in the
newly diagnosed patient with parkinsonism because its
pharmacological effect enhances the actions of endogenous
dopamine.
Selegiline is also used in conjunction with levodopa–
carbidopa in later-stage parkinsonism to reduce levodopa
dosage requirements and to minimize or delay
the onset of dyskinesias and motor fluctuations that
usually accompany long-term treatment with levodopa.
It has also been proposed that selegiline may slow the
progression of the disease by reducing the formation of
toxic free radicals produced during the metabolism of
dopamine. However, any neuroprotective effect
of selegiline in parkinsonian patients remains to be
established.
Most of the adverse reactions to selegiline are related
to actions of increased levels of dopamine, as discussed
earlier. At recommended doses, and unlike the
nonselective MAO inhibitors used in the treatment of
depression, selegiline has little effect on MAO-A and
therefore generally does not cause the hypertension associated
with the ingestion of tyramine-enriched foods. However, at doses higher than those
usually recommended, MAO-A may be inhibited,
which increases the risk of a tyramine reaction.
Selegiline should not be coadministered with tricyclic
antidepressants or selective serotonin uptake inhibitors
because of the possibility of a severe adverse drug reaction
(e.g., hyperpyrexia, agitation, delirium, coma). | [Synthesis]
Selegiline, N-methyl-N-(2-propinyl)-2-methyl-1-phenylethylamine (10.1.14),
is synthesized by the alkylation of (-)methyamphetamine (8.1.2.3) using propargyl�bromide [20¨C23].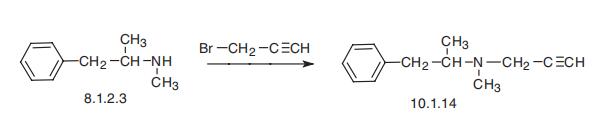
|
|
| Company Name: |
Embio Limited
|
| Tel: |
+91-9004390567 +91-9004390567 |
| Website: |
www.embio.co.in |
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|