| Identification | More | [Name]
Mitiglinide calcium | [CAS]
145525-41-3 | [Synonyms]
(as,3ar,7as)-octahydro-gamma-oxo-alpha-(phenylmethyl)-2h-isoindole-2-butanoic acid calcium salt dihydrate
calcium 2-benzyl-3-(cis-hexahydro-2-isoindolinylcarbonyl)propionate
mitiglinide calcium
MITIGLINIDE CALCIUM HYDRATE
(s)-2-benzyl-4-oxo-4-(cis-perhydroisoindol-2-yl)butyric acid calcium salt dihydrate
Monocalcium bis[(2S)-2-benzyl-3-(cis-hexahydro isoindolin-2-carbonyl)propionate]dihydrate
MITIGLINIDECALCIUM(FORR&DONLY)
MITIGLINIDE KAD-1229
Mitiglinide calcium
Mitiglinide calcium anhydrous | [EINECS(EC#)]
692-046-9 | [Molecular Formula]
(C19H24NO3)2.Ca | [MDL Number]
MFCD08458378 | [Molecular Weight]
668.88 | [MOL File]
145525-41-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [HS Code ]
2933.99.7500 |
| Questions And Answer | Back Directory | [Description]
Mitiglinide is the calcium salt form of Mitiglinide, which is a drug for the treatment of type II diabetes. It belongs to the meglitinide class blood glucose-lowering drug. Its mechanism of action is through stimulating the insulin secretion through closing the ATP-sensitive potassium channels in the pancreatic beta-cells. This process leads to depolarization, further stimulating the influx of calcium through the voltage-gated calcium channels. The high intracellular calcium level results in the exocytosis of insulin granules, alleviating the symptoms of type II diabetes.
| [References]
https://www.drugbank.ca/drugs/DB01252
https://en.wikipedia.org/wiki/Mitiglinide
|
| Hazard Information | Back Directory | [Originator]
Kissei (Japan) | [Uses]
Mitiglinide Calcium is a blood glucose-lowering drugs, stimulating insulin secretion by closing the ATP-sensitive K+ channels in pancreatic beta-cells | [Definition]
ChEBI: Kad 1229 is a polymer. | [Brand name]
Glufast | [Synthesis]
A number of publications and
patents have disclosed the syntheses of mitiglinide.
One of the syntheses describing the preparation of
mitiglinide using bis-activated esters to obtain a selective
mono amide is described in Scheme 8. The synthesis starts
with racemic 2-benzylsuccinic acid (51) which was resolved into its enantiomer using chiral amine salt formation and
crystallization. Out of several amines used, (R)-1-
phenylethylamine gave the best results for the chiral
resolution (99.5% ee, 19.5%). Acid 52 was treated with
thionyl chloride and triethylamine followed by Nhydroxysuccinamide
to give doubly activated ester 53
(97%). Treatment of this double ester 53 with
tetrahydroisoindoline (54) gave selectively mono amide
to di-amide in 99:1 ratio. Hydrolysis of the activated ester in
55 with water gave desired product 56 in 99% yield.
Subsequent conversion in two steps to the half calcium salt
provided mitiglinide calcium hydrate (VIII) in 91% yield.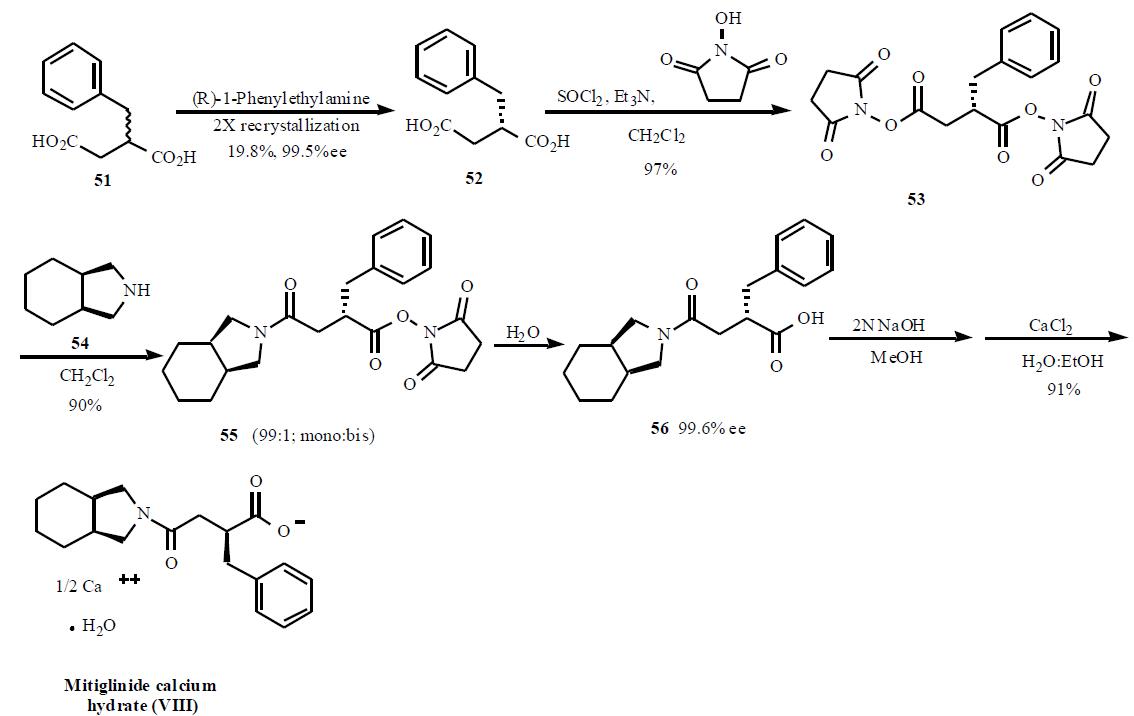
|
|
|