| Identification | More | [Name]
Potassium ethylxanthate | [CAS]
140-89-6 | [Synonyms]
ETHYLXANTHIC ACID POTASSIUM SALT
O-ETHYLXANTHIC ACID, POTASSIUM SALT
POTASSIUM ETHYLDITHIOCARBONATE
POTASSIUM ETHYL XANTHATE
POTASSIUM ETHYL XANTHOGENATE
POTASSIUM O-ETHYL DITHIOCARBONATE
POTASSIUM XANTHATE
POTASSIUM XANTHOGENATE
(o-ethyldithiocarbonato)potassium
carbonicacid,dithio-,o-ethylester,potassiumsalt
Carbonodithioicacid,O-ethylester,potassiumsalt
dithiocarbonicacid,o-ethylester,potassiumsalt
ethylpotassiumxanthate
ethylpotassiumxanthogenate
ethylxanthatedepotassium
ethyl-xanthicacipotassiumsalt
o-ethylpotassiumdithiocarbonate
O-Ethylxanticacid,potassiumsalt
z3
Potassium O-ethylxanthogenate | [EINECS(EC#)]
205-439-3 | [Molecular Formula]
C3H5KOS2 | [MDL Number]
MFCD00004931 | [Molecular Weight]
160.3 | [MOL File]
140-89-6.mol |
| Chemical Properties | Back Directory | [Definition]
Colorless or light-yellow crystals. Soluble in water and alcohol; insol-
uble in ether.
| [Appearance]
Light Green Solid | [Melting point ]
209-214 °C
| [Boiling point ]
462.37℃[at 101 325 Pa] | [bulk density]
400-600kg/m3 | [density ]
1,558 g/cm3 | [vapor pressure ]
0Pa at 25℃ | [Fp ]
96°C | [storage temp. ]
Store below +30°C. | [solubility ]
alcohol: soluble(lit.) | [form ]
Crystalline Powder | [color ]
Yellow | [PH]
9-10 (100g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. | [Water Solubility ]
Soluble in water. | [Detection Methods]
RT | [Merck ]
14,7696 | [BRN ]
3596974 | [InChIKey]
JCBJVAJGLKENNC-UHFFFAOYSA-M | [LogP]
-2.24 at 25℃ | [CAS DataBase Reference]
140-89-6(CAS DataBase Reference) | [EPA Substance Registry System]
140-89-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37:Wear suitable gloves .
S36:Wear suitable protective clothing . | [RIDADR ]
3342 | [WGK Germany ]
3 | [RTECS ]
FG1575000 | [TSCA ]
Yes | [HazardClass ]
4.2 | [PackingGroup ]
II | [HS Code ]
29309090 | [Toxicity]
LD50 orally in Rabbit: 1700 mg/kg |
| Hazard Information | Back Directory | [Hazard]
Toxic by ingestion.
| [Chemical Properties]
Light Green Solid | [Uses]
As reagent in analytical chemistry. | [Flammability and Explosibility]
Highlyflammable | [Purification Methods]
Crystallise it from absolute EtOH, ligroin/ethanol or acetone by adding Et2O. Wash it with ether, then dry it in a desiccator. Its solubility in Me2CO is 8% at 25o. [Beilstein 3 H 563, 3 I 196, 3 II 367, 3 III 1101, 3 IV 1274.] | [Mode of action]
Potassium ethylxanthate (KEtX) has long been utilized as a collector in the flotation of chalcocite (Cu2S), bornite (Cu5FeS4), chalcopyrite (CuFeS2) and galena (PbS). KEtX acts as an inhibitor of anodic corrosion of copper in acidic sodium chloride solutions. The collection efficiency of EtX? ion is ascribed to its interaction with mineral surfaces, possibly through a chemisorption bond. A similar interaction of EtX? ions with Pb has been found. It is interesting to note that the anodic oxidation of copper of KEtX to diethyl dixanthogen should be considered according to the following equations:
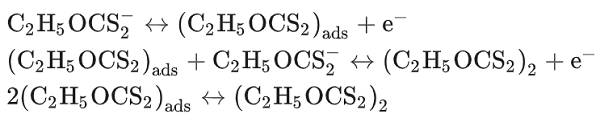
Diethyl dixanthoges does not diffuse into the bulk solution but forms a physically adsorbed layer. Interactions of EtX? ions with the Cu electrode surface are first manifested by the adsorption of EtX? ions, which is then followed, at higher positive potentials, by a growth of Cu(I)EtX film. On the other hand, the inhibitory effect of EtX? may probably be explained on the basis of stabilizing the +1 oxidation state of copper in the film against further oxidation and an increase in the hydrophobicity of the metal surface[1].
| [References]
[1] Scendo, M. “Potassium ethyl xanthate as corrosion inhibitor for copper in acidic chloride solutions.” Corrosion Science 47 7 (2005): Pages 1738-1749.
|
|
|