| Identification | More | [Name]
FMOC-N-ME-TYR(TBU)-OH | [CAS]
133373-24-7 | [Synonyms]
FMOC-METYR(TBU)-OH
FMOC-L-METYR(TBU)-OH
FMOC-N-ME-TYR(TBU)-OH
Fmoc-N-Me-L-Tyr(tBu)-OH
FMOC-N-ME-TYR(TBU)-OH 1 G
Fmoc-N-Me-Tyr(tBu)-OH 97%
FMOC-N-ME-TYR(TBU)-OH USP/EP/BP
FMOC-N-METHYL-O-T-BUTYL-L-TYROSINE
FMOC-N-METHYL-O-TERT-BUTYL-L-TYROSINE
N-FMoc-N-Methyl-O-tert-butyl-L-tyrosine
FMOC-N-ALPHA-METHYL-O-T-BUTYL-L-TYROSINE
fMoc-N-Me-tyr(tbu)-oh
Fmoc-N-methyl-O-tert-butyl-L-tyrosine≥ 98% (HPLC)
(9H-Fluoren-9-yl)MethOxy]Carbonyl N-Me-Tyr(tBu)-OH
O-(tert-Butyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-tyrosine
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-N-ALPHA-METHYL-O-T-BUTYL-L-TYROSINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-ALPHA-METHYL-O-T-BUTYL-L-TYROSINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-ALPHA-METHYL-O-TERT-BUTYL-L-TYROSINE
L-Tyrosine, O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N-methyl-
(S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-3-(4-tert-butoxyphenyl)propanoic acid
(2S)-3-[4-(tert-butoxy)phenyl]-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}(methyl)amino)propanoic acid | [Molecular Formula]
C29H31NO5 | [MDL Number]
MFCD02684471 | [Molecular Weight]
473.56 | [MOL File]
133373-24-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
186-191°C | [Boiling point ]
638.9±55.0 °C(Predicted) | [density ]
1.208 | [storage temp. ]
Store at 0-5°C | [form ]
powder or crystals | [pka]
2.98±0.10(Predicted) | [optical activity]
[α]22/D -49.0°, c = 0.5% in DMF | [InChIKey]
WTLSDEYZKFJXFT-SANMLTNESA-N | [SMILES]
C(O)(=O)[C@H](CC1=CC=C(OC(C)(C)C)C=C1)N(C(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O)C | [CAS DataBase Reference]
133373-24-7(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [General Description]
Fmoc-N-Me-Tyr(tBu)-OH is an Fmoc protected tyrosine derivative that is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Tyrosine is a very important amino acid - one of the few amino acids which is phosphorylated to vary the physical properties of the peptides, and is a precursor for the formation of iodinated thyroid hormones. The Fmoc group is typically removed with a base such as pyridine - an orthogonal de-protection strategy to the acid labilie Boc group. | [reaction suitability]
reaction type: Fmoc solid-phase peptide synthesis | [Synthesis]
(9H-Fluoren-9-yl)methyl carbonochloridate could be used as the starting material to synthesize Fmoc-N-Me-Tyr(tBu)-OH.
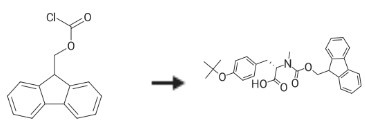
|
|
|