| Identification | More | [Name]
(1S,2R)-(-)-cis-1-Amino-2-indanol | [CAS]
126456-43-7 | [Synonyms]
(1S,2R)-1-AMINO-2,3-DIHYDRO-1H-INDEN-2-OL
(1S,2R)-(-)-1-AMINO-2-HYDROXYINDAN
(1S,2R)-1-AMINO-2-HYDROXYINDANE
(1S,2R)-(-)-1-AMINO-2-INDANOL
(1S,2R)-1-AMINO-2-INDANOL
(1S,2R)-1-AMINO-INDAN-2-OL
(1S,2R)-(-)-CIS-1-AMINO-2-HYDROXYINDANE
(1S,2R)-(-)-CIS-1-AMINO-2-INDANOL
(1S,2R)-CIS-1-AMINO-2-INDANOL
(1S,2R)-(-)-CIS-1-AMINOINDAN-2-OL
(1S,2R)-(+)-CIS-1-AMINOINDAN-2-OL
CIS-(1S,2R)-1-AMINO-2-INDANOL
cis-(1S)-Amino-(2R)-indanol
(Is-Cis)L-Amino-2,3-Dihydro-Lh-Inden-2-Ol,IndinavirSulphate,
(IS-cis) l-Amino-2,3-Dihydro-lH-Inden-2-ol
CIS-(1S,2R)-1-AMINO-INDANE-2-OL
(1S,2R)-(1)-cis-1-Amino-2-indanol
(1S,2R)-(-)-cis-1-Aminoindan-2-ol,98%
(1S,2R)-(-)-CIS-1-AMINO-2-INDANOL 99%
(1R,2S)-(-)-cis-a-Amino-2-indanol | [Molecular Formula]
C9H11NO | [MDL Number]
MFCD00216655 | [Molecular Weight]
149.19 | [MOL File]
126456-43-7.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
118-121 °C(lit.)
| [alpha ]
-62 º (c=0.5, CHCl3) | [Boiling point ]
270.27°C (rough estimate) | [density ]
1.0753 (rough estimate) | [refractive index ]
1.5760 (estimate) | [RTECS ]
NK7525500 | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
soluble in Methanol | [form ]
Powder | [pka]
14.79±0.40(Predicted) | [color ]
White to light beige | [optical activity]
[α]20/D 61°, c = 0.5 in chloroform | [Water Solubility ]
slightly soluble | [Sensitive ]
Air Sensitive | [BRN ]
4292559 | [InChIKey]
LOPKSXMQWBYUOI-BDAKNGLRSA-N | [CAS DataBase Reference]
126456-43-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
3259 | [WGK Germany ]
3
| [F ]
10-23 | [TSCA ]
No | [HazardClass ]
8 | [HS Code ]
29221990 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powder | [Uses]
1S,2R)-(-)-cis-1-Amino-2-indanol may be used to prepare:
- (-)-1,2,5,6-Tetrahydropyridine by reacting with methyl (E,E)-4-oxo-2-[(2,6,6-trimethylcyclohex-1-enyl)vinyl}but-2-enoate.
- Oxazaborolidine catalysts, which can catalyze the asymmetric reduction of aromatic ketones with high enantioselectivity.
- (RS,1S,2R)-(-)-2,4,6-Trimethylbenzenesulfinic acid 1-(2,4,6-trimethylbenzenesulfonylamino)indan-2-yl ester.
| [General Description]
(1S,2R)-(-)-cis-1-Amino-2-indanol is a main constituent of indinavir, a potent HIV (human immunodeficiency virus) protease inhibitor. |
| Questions And Answer | Back Directory | [Reaction]
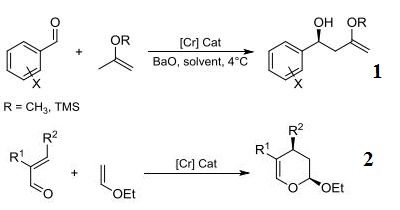
- Ligand component used in the chromium-catalyzed highly selective asymmetric ene reactions between aryl aldehydes and alkoxy- and silyloxyalkenes.
- Ligand component for the chromium-catalyzed highly enantioselective o inverse-demand hetero-Diels-Alder reactions of α,β-unsaturated aldehydes.
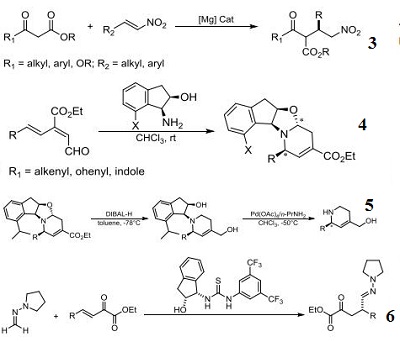
- Ligand component for the magnesium-catalyzed conjugate addition reaction of 1,3-dicarbonyl compounds to nitroalkenes.
- Component for stereoselective asymmetric 6π-azaelectrocyclization through the reaction between the (E)-3-
- carbonyl-2,4,6-trienal compounds and the (-)-7-alkyl-cis-1-amino-2-indanol derivatives.
- Ligand component for palladium-catalzyed asymmetric azaelectrocyclization for the preparation of 2,4-
- disubstituted chiral 1,2,5,6-tetrahydropyridines.
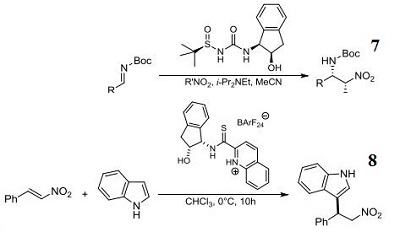
- Component for organocatalytic conjugate addition of formaldehyde N,N-dialkylhydrazones to β,γ -Unsaturated α-keto esters.
- N-Sulfinyl urea organocatalyst component for enantioselective aza-henry reaction.
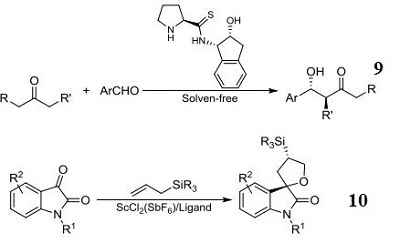
- Component for organocatalytic enantioselective additions of indoles to nitroalkenes.
|
|
|