| Identification | More | [Name]
Topotecan | [CAS]
123948-87-8 | [Synonyms]
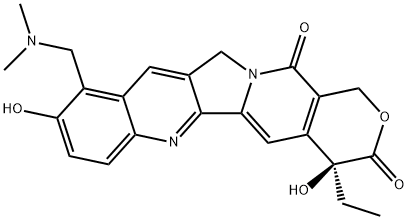
4-ethyl-4,9-dihydroxy-10-[(dimethylamino)methyl]-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione hydrochloride
4 N HCL CONSTANT BOILING
(4S)-10-[(DIMETHYLAMINO)METHYL]-4-ETHYL-4,9-DIHYDROXY-1H-PYRANO[3',4',6-7]INDOLIZINO[1,2-B]QUINOLINE-3,14(4H,12H)-DIONE
9-[(DIMETHYLAMINO)METHYL]-10-HYDROXY-(20S)-CAMPTOTHECIN, HCL
9-dimethylaminomethyl-10-hydroxycamptothecin hydrochloride salt
ACIDUM HYDROCHLORICUM
DEPURINATION SOLUTION
hycamptamine
hycamtin
HYDROCHLORIC ACID,1.1M
HYDROCHLORIC ACID, 1N
HYDROCHLORIC ACID, 2.00 N
HYDROCHLORIC ACID, 20 DEG BAUME
HYDROCHLORIC ACID, 2.50 N
HYDROCHLORIC ACID, 3.00 N
HYDROCHLORIC ACID, 5.00 N
HYDROCHLORIC ACID, 5N
HYDROCHLORIC ACID, 6.000N
HYDROCHLORIC ACID, 6.00 N
HYDROCHLORIC ACID, 6.0N | [EINECS(EC#)]
231-595-7 | [Molecular Formula]
C23H23N3O5 | [MDL Number]
MFCD00866235 | [Molecular Weight]
421.45 | [MOL File]
123948-87-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-white Cryst | [Melting point ]
−114 °C(lit.) | [Boiling point ]
>100 °C(lit.) | [density ]
1.2 g/mL at 25 °C(lit.)
| [vapor density ]
1.3 (vs air)
| [vapor pressure ]
613 psi ( 21.1 °C)
| [Fp ]
12 °C | [storage temp. ]
2-8°C
| [solubility ]
H2O: soluble
| [form ]
liquid
| [pka]
8.92±0.40(Predicted) | [color ]
yellow | [CAS DataBase Reference]
123948-87-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
T,C,F,Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R67:Vapors may cause drowsiness and dizziness.
R35:Causes severe burns.
R20:Harmful by inhalation.
R11:Highly Flammable.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
UN 3286 3/PG 2
| [WGK Germany ]
2
| [RTECS ]
MW4025000
| [F ]
3 | [HazardClass ]
8 | [PackingGroup ]
II | [Hazardous Substances Data]
123948-87-8(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
Topotecan was launched in the US for the second-line treatment of ovarian
cancer. It can be prepared in four steps from camptothecin and is a water soluble
derivative of the natural product with decreased toxicity. Unlike its chemical relative
irinotecan, topotecacan in not a prodrug and does not require bioactivation. It is an
inhibitor of topoisomerase I. Specifically, it inhibits the release of topoisomerase I
from DNA, where it relaxes supercoiled DNA, giving rise to single-strand breaks.
When the replication fork reaches this complex, double-stand breaks can occur. This
signals apoptosis and eventually gives rise to cell death. Evidence indicates
hycamtin is safe for people with impaired hepatic function. | [Chemical Properties]
Off-white Cryst | [Originator]
SmithKline Beecham (UK) | [Definition]
ChEBI: A pyranoindolizinoquinoline used as an antineoplastic agent. It is a derivative of camptothecin and works by binding to the topoisomerase I-DNA complex and preventing religation of these 328 single strand breaks. | [Manufacturing Process]
Camptothecin (CPT) - a compound isolated from the bark, leaves and fruit of
Camptotheca acuminate (Wall M. E. et al., J. Am. Chem. Soc. 88, 3888,
1966).
10-Hydroxycamptothecin (10-HCPT) was prepared by subjecting CPT (3.2 g
0.0092 mol), 0.8 g of Pt0 (prepared by pre-reduction of 8 g of amorphous
PtO2 in 80 ml of acetic acid for 1.5 h under 1 atm hydrogen pressure) and
acetic acid to 1 atm of H2 for 8.5 h after which theoretical amount of H2
absorbed (slightly more than 0.4 L) and uptake of H2 gets slowed down. The
reaction mixture was degassed under steam of helium and filtered through
celite and washed with acetic acid (20 ml). The resulting solution was treated
immediately with Pb(OAc)4 (6.4 g 0.014 mol) in portions and reaction
mixture, stirred vigorously under helium for 30 min. Gumy residue was
obtained on evaporation of solvent which was triturated with cold water (100
ml) to produce light brown solid. The solid was collected, washed with cold
water and air dried overnight when a mixture of 10-HCPT (44%), acetyl 10-
hydroxycamptothecin (10-AcHCPT, 26%) and unreacted CPT (32%) on HPLC
basis was obtained. This crude mixture was combined with 150 ml of 50%
acetic acid and heated under reflux conditions overnight. The reaction mixture
was cooled, concentrated to 20 ml and treated with cold water (100 ml) to
produce precipitate, which is filtered, washed with more cold water and dried
to afford 2.1 g of solid containing 10-HCPT (70%), 10-AcCPT (1.2%) and CPT
(21.3%) on the basis HPLC. Mixture was triturating with 0.5% aq HCl to
dissolve the water-soluble. When insoluble CPT was removed by filtration.
Water-soluble was extracted with chloroform and crystallized from boiling
solution of 20% of MeOH in CHCl3 by adding EtOAC dropwise until turbidity
appeared to obtain pure yellow 10-(HCPT), melting point 268°-270°C.
10-HCPT (0.364 g 0.01 mmol) and 40% aqueous dimethylamine (12 ml) was
added in dichloromethane (50 ml) in which anhydrous potassium carbonate
(2.17 g, 15 mmol) has been suspended. The reaction mixture was stirred at
room temperature for 5 h, then filtered and solid extracted with ethylacetate
(20 ml). The solvent is evaporated in vacuo giving a residue. The residue was
triturated with 0.5% aq HCl (50 ml) to dissolve the water-soluble adduct.
Water-soluble were partitioned with petroleum ether (3 times 50 ml) and
followed by ethylacetate (3 times 50 ml). The aqueous layer was lyophilized
as an off white hydrochloride salt of 9-[(dimethylamino)methyl]10-
hydroxy(20S)-camptothecin (topotecan hydrochloride) yield 0.236 g (65%). | [Brand name]
Hycamtin | [Therapeutic Function]
Antineoplastic | [reaction suitability]
reagent type: derivatization reagent
reaction type: Alkylations | [Biochem/physiol Actions]
Topotecan is a topoisomerase I inhibitor and an apoptosis inducer. It is a potent antineoplastic agent. | [Clinical Use]
Antineoplastic agent:
Treatment of metastatic ovarian, cervical and small cell lung cancer | [Drug interactions]
Potentially hazardous interactions with other drugs
None knownccccc | [Metabolism]
Topotecan undergoes reversible, pH-dependent hydrolysis of the active lactone moiety to the inactive hydroxyacid (carboxylate) form. A relatively small amount of topotecan is metabolised by hepatic microsomal enzymes to an active metabolite, N-demethyltopotecan; the clinical significance of this metabolite is not known. Excretion is via biliary and renal routes with 20-60
% excreted in the urine as topotecan or the open ring form. | [storage]
Store at -20°C,protect from light | [References]
[1] creemers gj1, lund b, verweij j. topoisomerase i inhibitors: topotecan and irenotecan. cancer treat rev. 1994 jan;20(1):73-96. |
| Questions And Answer | Back Directory | [Camptothecin anticancer drugs]
Camptothecin anticancer drugs are alkaloids or derivatives of them after the structure transformation raised from deciduous plant Davidia involucrata Camptotheca acuminate seed or root bark. Clinical application of them mainly includes camptothecin,hydroxycamptothecin, topotecan and irinotecan.
Topotecan is a water-soluble semi-synthetic derivative of camptothecin,it is a topoisomerase I inhibitor, inhibiting DNA single strand DNA scission reconnect to damage DNA, its cytotoxicity occurs in cancer cell division S phase, it is an s phase cell cycle specific drug . In vivo it is a two-compartment model,it can be quickly distributed to the liver, kidneys and other parts , of a single intravenous infusion 1.5mg/m2 every 30 minutes , t1/2αis 4.1 to 8.1 minutes, plasma protein binding rate is 6.6%~21.3%,it can go into the cerebrospinal fluid. Most of it is excreted by the kidneys, a small part of it is excreted by the bile, within 12 hours, 90% of it is excreted.Clearance rate of renal dysfunction decreases.it is clinically used for the treatment of small cell lung cancer (SCLC), and advanced ovarian cancer, because it can penetrate the blood-brain barrier ,it has a certain effect on the central nervous system cancer and brain metastases. Usage: every single drug dose 1.2mg/m2 intravenous infusion once a day, once every 5 days, 21 days is 1 cycle, there is the need to reduce the dose when in combination with other anticancer drugs. The main adverse events are hematologic toxicity, white blood cells, platelets and hemoglobin decrease; non-hematologic toxicity adverse events are loss of appetite, nausea, vomiting, hair loss, stomatitis, diarrhea, headache, fever, constipation, transient elevated transaminase; occasionally breathing difficulties, hematuria, andelectrocardiogram abnormalities.
Severe bone marrow suppression are hanged. Monitor the blood and adjust the dose in accordance with changes in blood during the treatment , if necessary, granulocyte colony stimulating factor (G-CSF), and transfusion of blood components may be given .
The above information is edited by the chemicalbook of Tian Ye.
| [Uses]
Anti-cancer drugs.It can be used alone for the treatment of initially or continuously ineffective chemotherapy metastatic ovarian cancer.
| [Production methods]
Compound (I) with 37% formaldehyde and 40% dimethylamine,after aminomethylation in acetic acid, produces the product. |
|
|