| Identification | Back Directory | [Name]
Etoposide | [CAS]
33419-42-0 | [Synonyms]
epe
epec
VP-16
e[qr]
nk171
Etopl
lastet
Eposin
Eto-Gry
Celltop
vepesid
Toposar
Vepeside
vepesidj
nsc141540
VP-16-213
ETOPOSIDE
VP 16-123
Zuyeyidal
nsc-141540
Etoposide COS
Etoposide, USP
trans-Etoposide
Etoposide USP26
Etoposide,Usp28
Etoposide BP2000
(5R,5aR,8aR,9S)-
Etoposide(α-tipe)
Etoposide (VP-16)
Etoposide (300 mg)
ETOPOSIDE (VEPESID)
ETOPOSIDE, USP 99+%
Etoposide , 98.0%(LC)
ETOPOSIDE, EP STANDARD
VP 16 (pharmaceutical)
ETOPOSIDE, USP STANDARD
Etoposide (FDA) USP24 BP98
ETOPOSIDE, MM(CRM STANDARD)
Epipodophyllotoxin VP 16213
Etoposide Resolution Mixture
Etoposide for system suitability
Etoposide4-O--D-Galactopyranoside
Demethylepipodophyllotoxin-beta-D-
Etoposide (contains ca. 5% Ethanol)
ETOPOSIDE 4-O-B-D-GALACTOPYRANOSIDE
Etoposide Resolution Mixture (30 mg)
ETOPOSIDE, RESOLUTION MIXTURE USP STANDARD
demethyl-epipodophyllotoxinethylideneglucoside
9-((4,6-O-Ethylidine-Beta-D-Glucopyranosyl)Oxy)-
DEMETHYLEPIPODOPHYLLOTOXIN-BETA-D-ETHYLIDENEGLUCOSIDE
4'-Demethylepipodophyllotoxin ethylidene-β-D-glucoside
EtoposideETOPOSIDE
CAS:33419-42-0
MES-012
BMY-40481, Etoposide 4'-Dihydrogenphosphate, VP-16-213
Etoposide-In combination with Cisplatin and Bleomycin
4-demethylepipodophyllotoxin-beta-d-ethylideneglucoside
4’-demethyl-epipodophyllotoxin-beta-d-ethyliden-glucosid
4’-demethylepipodophyllotoxinethylidene-beta-d-glucoside
4'-demethylepipodophyllotoxin 9-(4,6-o-ethylidene-β-d-glucopyranoside)
epipodophyllotoxin,4’-demethyl-,4,6-o-ethylidene-beta-d-glucopyranoside
4'-DEMETHYLEPIPODOPHYLLOTOXIN 9-(4,6-O-ETHYLIDENE-BETA-D-GLUCOPYRANOSIDE)
4-DESMETHYLEPIPODOPHYLLOTOXIN 9-(4,6-O-ETHYLIDENE)-BETA-D-GLUCOPYRANOSIDE
4'-Demethyl-1-O-[4,6-O-(ethylidene)-β-D-glucopyranosyl]epipodophyllotoxin
4'-demethylepipodophyllotoxin-(4,6-o-(r)-ethylidene-beta-d-glucopyranoside)
epipodophyllotoxin,4’-demethyl-,9-(4,6-o-ethylidene-beta-d-glucopyranoside)
4’-o-demethyl-1-o-(4,6-o-ethylidene-beta-d-glucopyranosyl)epipodophyllotoxin
xy-3,4-dimethyloxyphenyl)furo(3’,4’’:6,7)naptho-(2,3-d)-1,3-dioxol-6(5ah)-on
Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-β-D-glucopyranoside (8CI)
9-((4,6-o-ethylidine-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydro
GAL4 [(1-147) + VP16 (411-490)] from Saccharomyces cerevisiae human herpesvirus 2
Etoposide,4′-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-β-D-glucopyranoside), VP-16-213
9-((4,6-O-Ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one
(5R,5aα,9S)-9-[[4-O,6-O-[(R)-Ethylidene]-β-D-glucopyranosyl]oxy]-5,8,8aβ,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one
Furo3,4:6,7naphtho2,3-d-1,3-dioxol-6(5aH)-one, 9-4,6-O-(1R)-ethylidene-.beta.-D-glucopyranosyloxy-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-
[5R-[5α,5aβ,8aα,9β(R*)]]-9-[(4,6-0-Ethylidene-α-D-glucopyransoyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naptho[2,3-d]-1,3-dioxol-6-(5aH)-one
Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-[[4,6-O-(1R)-ethylidene-β-D-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-
[5R-[5A,5AB,8AA,9B(R*)]]-9-[(4,6-O-ETHYLIDENE-BETA-D-GLUCOPYRANSOYL)OXY]-5,8,8A,9-TETRAHYDRO-5-(4-HYDROXY-3,5-DIMETHOXYPHENYL)FURO[3',4':6,7]NAPTHO[2,3-D]-1,3-DIOXOL-6-(5AH)-ONE
Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-[(4,6-O-ethylidene-β-D-glucopyranosyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, [5R-[5α,5aβ,8aα,9β(R*)]]-
(-)-Etoposide, (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside
[5R-[5alpha,5abeta,8aalpha,9beta(R*)]]-9-[(4,6-O-Ethylidene-beta-D-glucopyranosyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one
(10R,11R,15R,16S)-16-{[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-Methyl-hexahydro-2H-pyrano[3,2-d][1,3]dioxin-6-yl]oxy}-10-(4-hydroxy-3,5-diMethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1,3(7),8-trien-12-one | [EINECS(EC#)]
251-509-1 | [Molecular Formula]
C29H32O13 | [MDL Number]
MFCD06799401 | [MOL File]
33419-42-0.mol | [Molecular Weight]
588.56 |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
236-251 °C (lit.) | [alpha ]
D20 -110.5° (c = 0.6 in chloroform) | [Boiling point ]
563.9°C (rough estimate) | [density ]
1.2966 (rough estimate) | [refractive index ]
-110.5 ° (C=0.6, CHCl3) | [storage temp. ]
Store at RT | [solubility ]
DMSO: 30 mg/mL
| [form ]
powder
| [pka]
9.8(at 25℃) | [color ]
white
| [biological source]
synthetic | [Water Solubility ]
Insoluble in water. | [Merck ]
3886 | [BCS Class]
4 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months | [IARC]
1 (Vol. 76, 100A) 2012, 1 (Vol. 76, 100A) 2012 | [EPA Substance Registry System]
Furo[3',4': 6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-[[4,6-O-(1R)-ethylidene-. beta.-D-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-(33419-42-0) |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Powder | [Usage]
A DNA topoisomerase II inhibitor. Semi-synthetic derivative of podophyllotoxin, related structurally to Teniposide. Antineoplastic. | [Usage]
An antitumur agent that complexes with topoisomerase II and DNA to enhance double-strand and single strand cleavage of DNA and reversible inhitit religation. Blocks the cell cycle in S-phase and G2-phase of the cell cycle. Induces apoptosis in nor | [Usage]
anticonvulsant | [Biological Activity]
Topoisomerase II inhibitor (IC 50 = 59.2 μ M). | [Description]
Etoposide is a plant alkaloid and an inhibitor of topoisomerase II (IC50 = 60.3 μM).1,2 It inhibits proliferation of a variety of adenocarcinoma cells (IC50s = 0.005-12,200 μM) and human umbilical vein endothelial (HUVEC) cells (IC50 = 0.249 μM).3 It reduces tumor growth in an Ma human embryonal carcinoma mouse xenograft model when administered at a dose of 25 mg/kg, an effect that is enhanced by concomitant administration of the immunosuppressant cyclosporin A (Item No. 12088).4 Etoposide also inhibits nuclear receptor coactivator 3 (IC50 = 2.48 μM).5 Formulations containing etoposide have been used in combination therapy in the treatment of cancer. | [Originator]
Etopos,Lemery,Mexico | [Definition]
ChEBI: Etoposide is a beta-D-glucoside, a furonaphthodioxole and an organic heterotetracyclic compound. It has a role as an antineoplastic agent and a DNA synthesis inhibitor. It is functionally related to a podophyllotoxin and a 4'-demethylepipodophyllotoxin. | [Indications]
Etoposide (VePesid) is a semisynthetic derivative of
podophyllotoxin that is produced in the roots of the
American mandrake, or May apple. Unlike podophyllotoxin
and vinca alkaloids, etoposide does not bind to microtubules.
It forms a complex with the enzyme topoisomerase
II, which results in a single-strand breakage of
DNA. It is most lethal to cells in the S- and G2-phases of
the cell cycle. Drug resistance to etoposide is thought to
be caused by decreased cellular drug accumulation.
Etoposide is most useful against testicular and ovarian
germ cell cancers, lymphomas, small cell lung cancers,
and acute myelogenous and lymphoblastic
leukemia.Toxicities include mild nausea, alopecia, allergic
reaction, phlebitis at the injection site, and bone
marrow toxicity. | [Manufacturing Process]
Preparation of 2,3-Di-O-dichloroacetyl-(4,6-O-ethylidene)-β-D-glucopyranose
(hydrogenolysis)
An over-dried 100 mL three-necked round bottom flask fitted with a stir bar,
low temperature thermometer, and H2 inlet was charged with 2,3-di-Oetoposide dichloroacetyl-1-O-benzyloxycarbonyl-(4,6-O-ethylidene)-β-D-glucopyranose
(1.8 mmol), in acetone (15-30% concentration) and 10% palladium on
activated carbon powder (0.2 mmol). The solution was stirred until uniform
and then cooled to -10°C to 0°C. After the reaction was over the catalyst was
filtered over sintered glass containing a plug of celite under reduced pressure.
The sintered glass is washed trice with one times the total reaction volume of
anhydrous acetone and the filtrates are pooled and then concentrated to
dryness under reduced pressure at a temperature close to 30°C. The crude
residue was dried under vacuum at ambient temperature and above
compound was thus obtained as white foam in 98% yield with a melting point
of 130°-132°C (from acetone).
Preparation of 4'-Demethyl-epi-podophyllotoxin-4-(2,3-di-O-dichloroacetyl-
4,6-O-ethylidene)-β-D-glucopyranoside
An oven-dried, three-neck 250 mL round bottom flask was fitted with a stir
bar, low temperature thermometer, septa and argon inlet, was introduced with
4'-demethyl-epi-podophyllotoxin (1 mmol), dry molecular sieve (1/16 δ
pellets) and anhydrous dichloromethane (20-50% concentration). 2-3-Di-Odichloroacetyl-(
4,6-O-ethylidene)-β-D-glucopyranose (1.7 mmol) in
dichloromethane (10-20% concentration) was added via double-ended needle.
The suspension was stirred until homogenous and then cooled to -40°C to -
60°C in an atmosphere of argon and in the absence of moisture. To the stirred
suspension was added via a syringe, trimethylsilyl trifluoromethane sulfonate
(2 mmol) over 30 minutes. The reaction was held at between -50°C and -
40°C for 30 minutes. The course of the coupling reaction was monitored by
thin layer chromatography. The suspension was allowed to warm to about -
30°C and filtered through a short celite/basic alumina column, eluting twice
with one times the total reaction volume of dichloromethane. The pooled
filtrate was evaporated under reduced pressure to yield the crude
intermediate product 4'-demethyl-epi-podophyllotoxin-4-(2,3-di-Odichloroacetyl-
4,6-O-ethylidene)-β-D-glucopyranose (yield 80%). This crude
product is used directly in the next step without any purification. A sample
was purified by the chromatraton for spectroscopic identification. The results
are as follows: m.p.: 242°-243°C (from methanol).
Preparation of 4-Demethyl-epi-podophyllotoxin-4-(4,6-O-ethylidene)-β-Dglucopyranose
(etoposide)
To 0.8 mmol of 4'-demethyl-epi-podophyllotoxin-4-(2,3-di-O-dichloroacetyl-
4,6-O-ethylidene)-β-D-glucopyranose in 10-25% concentration in methanol is
added 1.5 mmol of zinc acetate dihydrate. The reaction mixture is refluxed
with stirring under heating for 90 minutes. After completion of the reaction,
the mixture is cooled and the volume reduced to one third by rotary
evaporation under reduced pressure. Working up is effected by diluting the
reaction solution with 100 mL dichloromethane and 100 mL of water. The
aqueous phase was washed with 50 mL of dichloromethane. The combined
dichloromethane phases was washed twice with 50 mL water, 15 mL of
methanol was added to the first wash to prevent precipitation of etoposide.
The organic phase was dried over anhydrous sodium sulphate, filtered and
concentrated by evaporation under vacuum to an amorphous solid. This solid
was re-crystallized from methanol/n-pentane at -4°C to 0°C, thus obtaining
colorless amorphous powder of Etoposide (yield 68%), if the mother liquors
are treated the yield will be higher). Melting point: 256°-258°C.
Preparation of Etoposide employing 2,3-di-O-dichloroacetyl-(4,6-Oethylidene)-
β-D-glucopyranose and boron trifluoride etherate as catalyst
4'-Demethyl-epi-podophyllotoxin (1 mmol) and 2,3-di-O-dichloroacetyl-(4,6-
O-ethylidene)-β-D-glucopyranose (2 mmol) were introduced into dry
dichloromethane under anhydrous condition. When the temperature was
stabilized to -20°C to -30°C, boron trifluoride etherate (1.5 mmol) was added
slowly with stirring. Reaction was continued at this temperature and
monitored by thin layer chromatography. After the completion of the reaction
as evidenced by TLC, the solution was washed with water, dried over
anhydrous sodium sulfate and concentrated under reduced pressure to afford
the crude intermediate product 4'-demethyl-epi-podophyllotoxin-4-(2,3-di-Odichloroacetyl-
4,6-O-ethylidene)-β-D-glucopyranose. This crude product was
then converted to etoposide by following the procedure as above described.
The yield of final product etoposide was about 60%. | [Brand name]
Toposar(Sicor); Vepesid (Bristol-Myers Squibb). | [Therapeutic Function]
Antitumor, Antineoplastic | [General Description]
Etoposide is available in 50- and 100-mg capsules for oral useand in 100-mg vials for IV use. The agent is approved for usein testicular cancer and small cell lung cancer. It has alsobeen used in a wide variety of cancers including NSCLC,Hodgkin’s and non-Hodgkin’s disease, Kaposi sarcoma,acute lymphocytic leukemia, neuroblastoma, choriocarcinoma,and epithelial, ovarian, testicular, gastric, endometrial,and breast cancers. Etoposide is one of the few natural productderivatives that can be administered orally. When givenby this route, bioavailability is 50%. Administration by the IVroute is also utilized, and the drug is widely distributed whengiven by either route. The agent is highly protein bound(90%) primarily to albumin. Low albumin levels may lead toan increase in free drug and require a lowering of the dose.The drug does not penetrate the blood-brain barrier at normaldoses but does during high-dose therapy. Elimination occursprimarily in the urine with 30% to 40% of an IV dose appearingas unchanged drug. The elimination half-life is 5 to 10hours. Metabolism involves opening of the lactone ring togive the hydroxy acid as the major metabolite. Epimerizationoccurs at C-3 to give the cis-lactone, which may also undergohydrolysis to give the hydroxy acid. Glucuronidation and sulfationof the 4'-OH give products that are inactive. Activemetabolites are formed as a result of CYP3A4 mediated oxidative-O-demethylation of the 3'-methoxy group to give thecatechol followed by oxidation to give the quinone. The toxicitiesof etoposide include dose-limiting myelosuppression,produces nausea and vomiting in 30% to 40% of patients,which is more commonly seen when the drug is administeredorally. The agent also produces anorexia, alopecia, mucositis,and hypersensitivity reactions that may be caused by etoposideor Cremophor EL (polyoxyethylated castor oil), which isused as a vehicle for IV administration of the drug. Leukemia,especially acute myelogenous leukemia, has been associatedwith the drugs’ ability to produce strand breaks with resultanttranslocation of genetic material. The leukemias are generallyseen 5 to 8 years posttreatment and have been associated withtranslocation of several different genes resulting in breakpointsaround the mixed lineage leukemia (MLL) gene.Transcription and translation of this altered DNA giveschimeric proteins, which form partly from the translocatedgene and partly from the MLL gene. Exactly how thesechimeric proteins lead to leukemia is not known, but similaralterations are seen with other topoisomerase inhibitors. | [Biochem/physiol Actions]
Etoposide is an antitumor agent that complexes with topoisomerase II and DNA to enhance double-strand and single-strand cleavage of DNA and reversibly inhibit religation. Blocks the cell cycle in in S-phase and G2-phase of the cell cycle; induces apoptosis in normal and tumor cell lines; inhibits synthesis of the oncoprotein Mdm2 and induces apoptosis in tumor lines that overexpress Mdm2. | [Clinical Use]
Etoposide is utilized in the treatment of small cell lung cancer and in combination with other agents in refractory testicular cancer. | [Synthesis]
Etoposide, [[5R-(5|á,5a|?,8a|á,9|?)]-9-[4,6-O-ethylidene-|?-D-glucopyranosyl)
oxy]-] 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3�,4�: 6,7]-naphtho[2,3-
d]-1,3-dioxol-6(5aH)-one (30.4.5), is made from 4�-desmethylepipodophyllotoxin (30.4.3),
the phenolic group of which being previously protected by benzyl chloroformate, which
makes 4�-carbobenzyloxy-4�-desmethylepipodophyllotoxin (30.4.3). Next, the hydroxyl
group at position C9 is esterified with 4,6-O-ethylyden-2,3-di-O-acetyl-|?-D-glucopyranose in
the presence of boron trifluoride to make the corresponding glucopyranoside 30.4.4.
Removing the acetyl group in the glucopyranosyl part of the molecule using zinc acetate in
sodium methoxide, and also removing the benzyloxycarbonyl protection by hydrogenation
using a palladium on carbon catalyst gives the desired etoposide (30.4.5).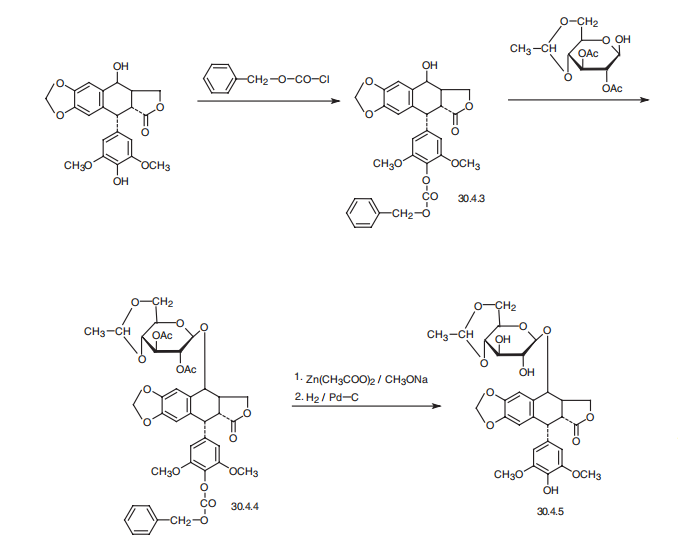
| [Metabolism]
The drug is more than 96% protein bound, undergoes biphasic elimination, and has a terminal half-life of 4 to 11 hours. Approximately 35 to 45% of a dose is eliminated via the kidneys, with less than 6% excreted in feces. The drug should be used with caution in patients with renal or liver disease. | [storage]
Store at RT |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
45-22-36/37/38 | [Safety Statements ]
53-45-36/37-26 | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
KC0190000
| [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
29389000 | [Safety Profile]
Poison by ingestion,
intraperitoneal, intravenous, and
subcutaneous routes. An experimental
teratogen. Human systemic effects by
ingestion and inhalation: agranulocytosis,
aplastic anemia, and other changes in bone
marrow. Experimental reproductive effects.
Human mutation data reported. When
heated to decomposition it emits acrid
smoke and fumes. | [Hazardous Substances Data]
33419-42-0(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 147mg/kg |
| Questions And Answer | Back Directory | [Pharmacological effects]
The chemical name of etoposide is 9-(4, 6-O-ethylidene-β-D-glucopyranoside)-4'-demethyl-epipodophyllotoxin. It is an off-white crystalline powder and is odorless. Upon being exposed to light, heat, its color is easy to change. It is also hydroscopic. It is almost insoluble in water, slight soluble in methanol, dimethyl sulfoxide and also ethanol.
Etoposide is the newly semi-synthetic derivative of epipodophyllotoxin and belongs to mitotic inhibitors which can make the cells be stalled in the mid-mitosis stage. It is a cell cycle specific anticancer drug. This product can act on the DNA topoisomerase II (Topo II), to form a "Drug-enzyme-DNA" complex, preventing Topo II from participating in DNA repair, resulting in the stallation of DNA replication, thereby inhibiting the proliferation of tumor cell(IC 50 = 59.2 μ M). It mainly takes effects on S phase, G2 phase cells, and caused cell arrest in the G2 phase. The experimental study has found that the complex can be reversed with the elimination of drug. In that case, Top II will become free again so the damaged DNA get repair again, reducing its anti-tumor effect. Therefore, extending the treatment time can enhance the anti-tumor activity. It is mainly used for the treatment of small cell lung cancer, malignant lymphoma, malignant germ cell tumors, and leukemia and also has certain efficacy on treating neuroblastoma, rhabdomyosarcoma, ovarian cancer, non-small cell lung cancer, stomach cancer and esophageal cancer.
This product has a bioavailability of 48% (25% to 74%) after oral administration. The plasma concentration can reach peak at 0.5 to 4 hours after taking this drug. After intravenous injection of this product, the plasma concentration of this drug exhibits biphasic elimination with the half-life of α phase being (1.4 ± 0.4) h and half-life of β phase being (5.7 ± 1.8) hours. The plasma protein binding rate is 74% to 90% with the highest concentration being found in intestine, liver, and kidney while the drug concentration in the cerebrospinal liquid is only 2% to 10% of that in the blood. It is primarily subject to renal excretion with 45% being excreted in the urine at 72 hours after the administration wherein prototype accounts for two-thirds and metabolites account for 15%. 1.5% to 16% of the drug is excreted through from faeces via the bile.
| [Side effects]
1. Over-rapid intravenous infusion rate (less than 30 minutes for the first time of administration) may cause rash, chills, fever, bronchospasm, dyspnea and other allergic reactions.
2. The drug can cause obvious myelosuppression reaction including anemia, leukopenia and thrombocytopenia. This frequently occurs in 7 to 14 days after treatment and can recover after 20 days of stopping administration. Severe neutropenia is the dose-limiting toxicity of the drug.
3. There may be loss of appetite, nausea, vomiting, stomatitis, diarrhea, abdominal pain and constipation. Liver toxicity is rare and may be accompanied with increased level of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase and bilirubin.
4. There may be occasional elevated level of blood urea nitrogen.
5. There may be dizziness, fatigue and tiredness with occasional numbness, headaches and so on; there may be heart palpitations, ECG changes, hypotension; interstitial pneumonia may also occur; hair loss is also common.

Figure 1 the structural formula of etoposide
| [Contraindications]
1. Patients of significantly lower amount of white blood cells and platelets should be disabled.
2. Patients of heart, liver and kidney dysfunction should be disabled.
3. Pregnant women and lactating women should be disabled.
4. Patients allergic to this drug should be disabled.
| [Uses]
It is used as anti-cancer drug mainly used for the treatment of small cell lung cancer, testicular cancer, malignant lymphoma and acute leukemia. It also has certain efficacy in treating neuroblastoma, rhabdomyosarcoma, ovarian cancer, non-small cell lung cancer, stomach cancer and breast cancer.
| [Usage and Dosage]
1. Oral: single-administration; daily: 60~100mg /m2; continuously apply for 10 days and repeat every 3 to 4 weeks. For combination chemotherapy, apply 50 mg/m2 per day and continue to take 3 or 5 days.
2. Intravenous infusion: Use sodium chloride injection for dilute this product of required amount (this drug is instable in 5% glucose injection and can form a fine precipitate). The concentration should not be more than 0.25 mg/ml with the intravenous infusion time being not less than 30 minutes.
Solid tumors: 60~100mg/m2 per day; continue for 3 to 5 days with repeating the medication every 3 to 4 weeks.
Leukemia: 60~100mg/m2 per day; apply for 5 consecutive days; repeat the medication at certain interval according to the blood condition.
Common pediatric dose: for intravenous infusion, administer based on volume/surface area 100~150mg/m2 for continuous 3 to 4 days.
| [Precautions]
1. This product is not suitable for intravenous injection and the intravenous infusion rate should not be too fast and should at least last for half an hour, otherwise it can easily lead to hypotension, laryngeal spasm and other allergic reactions.
2. Don’t choose chest, abdomen and intrathecal injection for administration.
3. During the medication period, the patients should be subject be regular investigation on the peripheral blood condition as well as liver and kidney function.
4. This product should be administrated immediately after dilution. If precipitate occurs, it should be strictly prohibited.
5. This product can cause reproductive toxicity and teratogenicity to animals and can be excreted through breast milk. FDA provided the pregnancy safety of this drug being classified as D class.
This information is edited by Xiongfeng Dai from Chemicalbook.
| [Drug Interactions]
1. Because this product has significant bone marrow suppression effect and should be taken care of when be used in combination with other anticancer drugs.
2. This product can inhibit the body's immune defense mechanism, so that vaccination is not able to stimulate the body to produce antibodies.
3. Within 3 months after the end of chemotherapy, it is not recommended for applying the vaccine virus.
4. This product has a high binding rate to the plasma protein and therefore, the drug bound to plasma protein can affect the excretion of this product.
|
| Questions and Answers (Q&A) | Back Directory | [References]
Hande, K. R. "Etoposide: four decades of development of a topoisomerase II inhibitor." European Journal of Cancer34.10(1998):1514.
Noda, K, et al. "Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer." New England Journal of Medicine 346.2(2002):85-91.
|
|
|