| Identification | More | [Name]
Zafirlukast | [CAS]
107753-78-6 | [Synonyms]
cyclopentyl [3-[[2-methoxy-4-[(2-methylphenyl)sulfonylcarbamoyl]phenyl]methyl]-1-methyl-indol-5-yl]aminoformate
cyclopentyl n-[3-{[2-methoxy-4-[(2-methylphenyl) sulfonylcarbamoyl]phenyl]methyl}-1-methyl-indol-5-yl]carbamate
ZAFIRLUKAST
Zafirlukast Tablet
[3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamic Acid Cyclopentyl Ester, ICI-2204219, Accolate
Cyclopentyl [3-[[2-methoxy-4-[(2-methylphenyl)sulfonylcarbamoyl]phenyl]methyl]-1-methyl-indol-5-yl]aminoformate
Cyclopentyl N-[3-{[2-methoxy-4-[(2-methylphenyl) sulfonylcarbamoyl]phenyl]methyl}-1-methyl-indol-5-yl]carbamate
[3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-y1]carbamic acid eyclopentyl ester
Accolate
ICI-204219
Carbamic acid, [3-[[2-methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]-, cyclopentyl ester
Cyclopentyl 3-[2-methoxy-4-[(o-tolylsulfonyl)carbamoyl]benzyl]-1-methylindole-5-carbamate
[3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamic acid cyclopentyl ester
4-[5-(Cyclopentyloxycarbonylamino)-1-methyl-1H-indol-3-ylmethyl]-3-methoxy-N-(2-methylphenylsulfonyl)benzamide
IC-204219 | [Molecular Formula]
C31H33N3O6S | [MDL Number]
MFCD00864775 | [Molecular Weight]
575.68 | [MOL File]
107753-78-6.mol |
| Questions And Answer | Back Directory | [Synthesis]
Reddy et. al. reported a total synthesis of zafirlukast 15 by coupling bromide 24, derived from commercially available 3-methoxy-4-methylbenzoic acid 23 with 1-methyl-5- nitroindole 25. Bromination of 23 with DBDMH in the presence AIBN in CHCl3, afforded bromomethylbenzoic acid derivative 24 in 81% yield. Alkylation of indole 25 with bromide 24 in dioxane mediated by ZnBr2 at 60-65 °C furnished indole 26 in 62% yield. Acid 26 was coupled with o-toluene sulfonamide 22 using DCC in the presence of DMAP as a catalyst to give sulfonamide 27 in 63% yield. Catalytic hydrogenation of 27 using Raney Ni in MeOH smoothly afforded amine, which upon acylation with cyclopentyl chloroformate 20 yielded the target molecule 15 in 89% yield.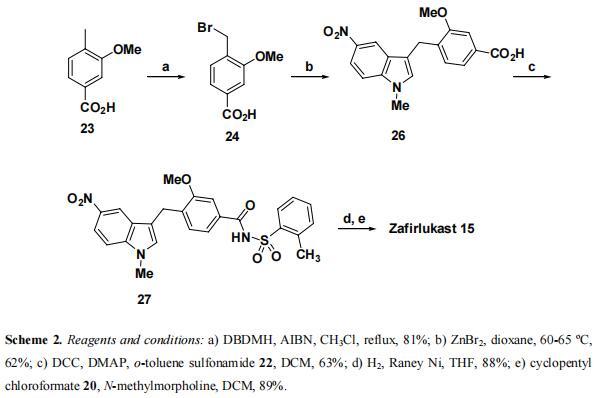 | [Anti-leukotriene antiasthmatic drugs]
Zafirlukast is a highly selective and long-acting oral lys-LTs antagonist. Leukotrienes play an important role in the pathogenesis of asthma. These substances are peptides, being capable of selectively binding to LTC4, LTD4 and LTE4 receptor for antagonism of its effect, reducing the symptoms of asthma and improving lung function. This product is both a preventive agent of inflammation caused by asthma (being antagonistic to leukotriene proinflammatory activity) and a relief drug for bronchial asthma (be antagonistic to leukotriene induced bronchial smooth muscle contraction). The use of this product does not change the smooth muscle response to β2 receptors and has good efficacy in the treatment of bronchoconstriction caused by antigen, aspirin, exercise and cold air, being able to reduce the dosage of hormone and β2 receptor agonist.
| [Mechanism of Action]
Zafirlukast is potent oral polypeptide trinaphthalene receptor antagonist of the slow response substances of supersensitive reactions such as LTC4, LTD4 and LTE4. It belongs to competitive inhibitor and has high selectivity, which can effectively prevent trinaphthalene-induced increased vascular permeability, tracheal edema and eosinophil infiltration.
Five-day doses of the drug reduce cellular and non-cellular inflammatory substances in the trachea due to antigen stimulation. In the bronchoalveolar lavage fluid of 48h after antigen challenge, the drug can inhibit the increase of eosinophils, lymphocytes and tissue cells, as well as reduce the peroxide produced by alveolar macrophage stimulation. It can inhibit antigen-induced airway hyperresponsiveness and platelet-activating factor-induced bronchospasm.
Long-term use of this drug can reduce the airway sensitivity to methacholine.
Asthma patients has a 10 times higher sensitivity to leukotriene D4 than the average person with a single oral dose of the drug increasing the tolerance of asthma patients to inhaled LTD4 by 100 times with significant protective effect within 12 and 24h t. It can inhibit bronchospasm caused by various kinds of stimuli (such as sulfur dioxide, exercise and cold air), reducing early and late inflammatory reactions caused by various causes such as pollen, cat hair, ragweed and mixed antigens. For some patients, the drug can completely prevent the movement and allergens caused by asthma attacks. The drug can be used to maintain first-line treatment of asthma patients who are on demand for beta-agonist therapy but are not adequately controlled. For patients with clinical symptoms, the drug can relieve symptoms (reduce the symptoms of diurnal asthma), improve lung function, reduce the amount of β-agonists and the incidence of asthma exacerbations.
Clinical study finds that medication within 2h, before the plasma concentration of the drug has reached the peak; it can already have a significant first-dose effect on basal bronchial tension. The symptoms of asthma can be initially alleviated within 1 week, mostly occurring in the first few days. Oral administration has a Tmax of 3h with twice daily (30 ~ 80mg, bid), we can see the plasma accumulation of Zafirlukast change from undetectable level to 2.9 times of the first dose with an average of 1.45 and median 1.27. The PBP was 99% and the t1/2 was 10h. Zafirlukast has a pharmacokinetic in healthy people compared with asthma youth and adults. When administered by body weight, there is no gender difference in the drug's metabolism. As with food, most patients (75%) have reduced bioavailability. Metabolism is complete, mainly excretion (89%) and urine (10%) excretion.
| [Pharmacokinetics]
This product is highly selective with only acting on leukotriene receptors. It does not affect prostaglandins, thromboxanes, cholinergic and histamine receptors. Oral absorption is good with plasma concentration reaching peak at about 3 hours. Medication within 2 hour has 10% undergoing urinary excretion and 89% undergoing stool excretion and half-life of about 10 hours. Pharmacokinetics in the normal population and kidney damage patients has no significant difference. The bioavailability of most patients can decrease by 40% if co-administrated with food.
| [Indications]
Clinically suitable for the prevention and treatment of chronic mild to moderate asthma, especially for patients with aspirin or aspirin asthma, or with upper respiratory tract diseases (such as nasal polyps, allergic rhinitis), but not for the treatment of acute asthma. It is suitable for hormone-resistant asthma or asthmatic patients who refuse use of hormone. In cases of serious asthma, additional administration with this product can maintain control of asthma attacks or to reduce the amount of hormones.
| [Medicine interactions]
- In combination with aspirin, the plasma concentration of the drug can be increased by about 45%, but not to the corresponding clinical effect.
- Combination with erythromycin, theophylline and terfenadine can cause the reduction of plasma concentrations (30%, 40% and 54%).
- Combination with warfarin can extend the maximum prothrombin time by about 35%. Therefore, upon the combination of the above two drugs, the patients should be closely monitored of prothrombin time.
| [Adverse reactions]
It can cause headache, gastrointestinal reactions, elevated aminotransferases, pharyngitis, rhinitis, urticaria, angina edema, rash, blisters and so on. A larger dose of medication can increase the incidence of hepatocellular carcinoma, histiocytic sarcoma and bladder cancer.
| [Precautions]
- Patients allergic to this product and children under 12 years of age should be disabled.
- Pregnant women, lactating women and liver damage should not use.
- Patients of cirrhosis, liver function damage use with caution.
- This product does not apply to relieve bronchial spasm during acute exacerbation of asthma, nor should it be used as an alternative to inhaled or oral glucocorticoid therapy.
- The product of cytochrome P4502C9 enzyme system has inhibitory effect.
- Information on the pharmacological Effects, pharmacokinetics, indications, drug interactions, and adverse Reactions Related to anti-asthma.
| [Synthesis]
5-nitroindole(I, 5.0 g, 30.8 mmol) and methyl-4-(bromomethyl)-3-methoxybenzoate (II, 7.99 g, 30.8 mmol) were dissolved in 30 ml of dioxane, Silver oxide (7.15 g, 30.8 mmol) was added under nitrogen protection and stirring and heated at 60 ° C for 20 h. The solvent was distilled off under reduced pressure and 50 ml of ethyl acetate was added. Filtrate; concentrate of the filtrate, followed by chromatography with eluting by 3: 7 ethyl acetate-hexanes. The resulting yellow oil was crystallized from methylene chloride-hexane to give 4.6 g of Compound (III) in 45% yield, m.p. 153-155 ° C.
Compound (III) (0.44 g, 1.29 mmol) was added to a suspension of free sodium hydride (0.031 g, 1.29 mmol) in 100 ml of dry tetrahydrofuran under nitrogen atmosphere, and add after iodomethane (0.18 g, 1.29mmo1) after 10 minutes for reaction of 30 min. Pour 30 ml of 1 mol / L hydrochloric acid and extract with ethyl acetate (2 x 50 ml). The extract was washed with saturated brine, dried and concentrated. The residue was separated by chromatography and eluted with hexane-methylene chloride-ethyl acetate 50: 45: 5. The resulting yellow oil was crystallized from a mixture of dichloromethane and hexane to give 0.33 g of compound (IV) Rate of 72%, melting point 144 ~ 146 ℃.
Compound (IV) (0.56 g, 1.57 mmol) was dissolved in 30 ml of tetrahydrofuran and 0.1 g of 10% palladium-carbon catalyst was added and hydrogenated at 0.345 MPa for 2 h. Filtration and concentration of the filtrate followed by purification by chromatography gave 1 g of ethyl acetate-hexane as eluent to give 0.5 g of compound (V) in a yield of 98%.
Compound (V) (0.25 g, 0.77 mmol) and N-methylmorpholine (0.77 mmol) were dissolved in 3 mL of dichloromethane; cyclopentyl chloroformate (0.11 g, 0.77 mmol) was added under nitrogen and stirred for 2 h. Pour 1mol / L hydrochloric acid, extracted with ethyl acetate. The extract was washed with saturated brine, dried and concentrated. The remaining viscous oil was purified by chromatography eluting with 3: 7 ethyl acetate-hexane to give 0.25 g of compound (VI) in 74% yield.
Compound (VI) is hydrolyzed with lithium hydroxide at room temperature to give compound (VII). (VII) and o-methylbenzenesulfonamide, in the presence of 1- [3- (dimethylamino) propyl] -3-ethylcarbodiimide hydrochloride and 4-(dimethylamino) pyridine, are condensed in methylene chloride, to give zafirlukast; yield 69%, mp 138-140 ° C.
|
| Hazard Information | Back Directory | [Description]
Accolate was launched in Ireland, Finland and the US for treatment of
asthma. Prepared via an eight step synthesis from methyl 3-methoxy-4-
methylbenzoate, zafirlukast acts as a LTD4 antagonist and is the first compound of a
new class of drugs. LTC4, LTD4 and LTE4 were determined to be the constituents of
the slow-reacting substance of anaphylaxis (SRS-A) which was found to induce
asthma effects (bronchoconstriction, increased vascular permeability resulting in
edema, cellular infiltration of airway tissues and decreased mucociliary transport).
Thus an inhibitor of their synthesis would at least attenuate these symptoms.
Zafirlukast binds to the CysLT receptor LT-1 and blocks the effect of LTC4, LTD4 and
LTE4. The drug is an oral twice daily formulation that reversed an LTD4 challenge,
attenuated the response of platelet-activating factor (PAF), an allergen and cold air
challenge and exercise-induced asthma. | [Chemical Properties]
Off-white to pale pink crystalline solid | [Originator]
Zeneca (UK) | [Uses]
Zafirlukast has been used to stimulate pancreatic β cell line (MIN6) and pancreatic islets for insulin secretion assay. It may be used as an adenosine triphosphate-binding cassette transporter (ABCG2) inhibitor in MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cytotoxicity assay in human embryonic kidney cells (HEK293) and in Kirby-Bauer disc diffusion assays, bactericidal activity and minimal inhibitory concentration (mIC) assay against M. smegmatis | [Uses]
A potent, selective and orally active CysLT receptor antagonist. Leukotriene D4 antagonist. Used as an antiasthmatic | [Uses]
antiasthmatic;leukotriene receptor antagonist (LTRA) | [Definition]
ChEBI: Zafirlukast is a member of indoles, a carbamate ester and a N-sulfonylcarboxamide. It has a role as an anti-asthmatic agent and a leukotriene antagonist. | [Manufacturing Process]
6-Nitroindol with 4-bromobenzyl-3-methoxy-benzoic acid methyl ester gives in
presence of silver oxide catalyst the diarilmethane 2-(2,4-dimethoxy-benzyl)-
5-nitro-1H-indole. The indole nitrogen is then converted to its anion with
sodium hydride; treatment with methyl iodide gives the corresponding N-
methyl derivative. Catalitic hydrogenation then converts the nitro group to the
amine to give 4-(6-amino-1H-inden-2-ylmethyl)-3-methoxy-benzoic acid
methyl ester. Acylation of that amine with cyclophentylchloroformate then
gives the urethane [2-(2,4-dimethoxy-benzyl)-1-methyl-1H-indol-5-yl]-
carbamic acid cyclopentyl ester. The benzoate ester is then selectively cleaved
with lithium hydroxide in dimethyl formamide to the corresponding carboxylic
acid. The intermediate is converted to the acyl sulfonamide by coupling with
ortho-toluenesulfonamide to give [2-(2-methoxy-4-o-
tolylmethanesulfonylaminocarbonyl-benzyl)-1-methyl-1H-indol-5-yl]-carbamic
acid cyclopentyl ester (zafirlucast). | [Brand name]
Accolate (AstraZeneca). | [Therapeutic Function]
Anti-asthmatic | [Acquired resistance]
Zafirlukast inhibits CYP3A4 and
CYP2C9 in concentrations equivalent to clinical plasma levels and, therefore, should be used
with caution in patients taking drugs metabolized by these enzymes. Specifically,
coadministration with warfarin results in a significant increase in prothrombin time. Other drugs
metabolized by CYP2C9 are phenytoin and carbamazepine. In
addition, CYP3A4-metabolized drugs are cyclosporine, cisapride, and the dihydropyridine class of
calcium channel blockers. Of particular interest is the fact that aspirin increases the plasma
levels of zafirlukast, and theophylline decreases the plasma levels of zafirlukast. Care should be
taken when coadministering with erythromycin, because this decreases the bioavailability of
zafirlukast. | [Biochem/physiol Actions]
Zafirlukast plays a key role in alleviating mucus and airway constriction in asthma based inflammatory response. It regulates pancreatic β cells for the secretion of insulin and this functionality is interlinked to the calcium based phosphorylation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase B (AKT). Zafirlukast has inhibitory potential on ATP-binding cassette (ABC) transporters and reverses the multidrug resistance function of ATP-binding cassette super-family G member 2 (ABCG2). Zafirlukast inhibits mycobacterial nucleoid-associated protein Lsr2 and halts the growth of Mycobacteria. | [Clinical Use]
Zafirlukast is an indole derivative with a sulfonamide group that fulfills the need for an ionizable
moiety on the pharmacophore. A large number of analogues have been prepared; however, they
all resulted in a decrease in antagonist activity. Zafirlukast, like montelukast, is a selective
antagonist for the cysLT1 receptor and antagonizes the bronchoconstrictive effects of all
leukotrienes (LTC4, LTD4, and LTE4). | [Veterinary Drugs and Treatments]
While zafirlukast potentially could be useful in treating feline asthma,
including allowing dose reductions of corticosteroid therapy,
its efficacy has been disappointing to this point and most do not
recommend its use. Potentially, it could be of benefit in allergymediated
(where leukotrienes play a role) dermatologic conditions, such as atopy in dogs, but evidence has been that it is not
very effective. | [Drug interactions]
Potentially hazardous interactions with other drugs
Aminophylline: possibly increases aminophylline
concentration; zafirlukast concentration reduced.
Analgesics: concentration increased by aspirin.
Antibacterials: concentration reduced by
erythromycin.
Anticoagulants: may enhance the effects of warfarin.
Theophylline: possibly increases theophylline
concentration; zafirlukast concentration reduced. | [Metabolism]
Zafirlukast is well absorbed orally; however, food will
decrease its absorption by as much as 40%. Zafirlukast is primarily metabolized in the liver by
CYP2C9 and CYP3A4 to hydroxylated metabolites. Zafirlukast also has been shown to
undergo carbamate hydrolysis, followed by N-acetylation. Additionally, zafirlukast in known to
produce an idiosyncratic hepatotoxicity in susceptible patients. This is appears to result from the
formation of an electrophilic α,β-unsaturated iminium intermediate evidenced by the formation of
a glutathione adduct on the methylene carbon bridging the indole ring to the methoxybenzene
moiety of the molecule. More than 90% if its metabolites are
excreted in the feces, with the remaining found in the urine. | [References]
1) Adkins and Brogden, (1998), Zafirlukast. A review of its pharmacology and therapeutic potential in the management of asthma; Drugs, 55 121
2) Woszczek et al., (2010), Concentration-dependent noncysteinyl leukotriene type 1 receptor-mediated inhibitory activity of leukotriene receptor antagonists; J. Immunol. 184 2218
3) Wyttenbach & Kuentz (2017), Glass-forming ability of compounds in marketed amorphous drug products; Eur. J. Pharm. and Biopharm., 112 204 [Focus Citation] |
|
|