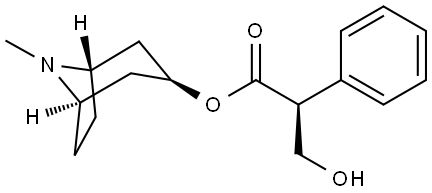
| Identification | More | [Name]
L-Hyoscyamine | [CAS]
101-31-5 | [Synonyms]
[3(S)-ENDO]-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL ESTER, ALPHA-(HYDROXYMETHYL)-BENZENEACETIC ACID
BENZENEACETIC ACID, ALPHA-(HYDROXYMETHYL)-, 8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL ESTER, [3(S)-ENDO]
DATURINE
(-)-HYOSCYAMINE
HYOSCYAMINE
L-DATURINE
L-DUBOISINE
L-HYOSCYAMINE
L-TROPINE TROPATE
(-)-hyoscyamin
(-)-tropicaciesterwithtropine
(3(s)-endo)-leste
(S)-(-)-Hyoscyamine
[3(s)-endo]-leste
1-Hyoscyamine
alpha-(hydroxymethyl)-benzeneaceticaci8-methyl-8-azabicyclo(3.2.1)oct-3-y
alpha-(hydroxymethyl)-benzeneaceticaci8-methyl-8-azabicyclo[3.2.1]oct-3-y
Cystospaz
Duboisine
Hyocyamine | [EINECS(EC#)]
202-933-0 | [Molecular Formula]
C17H23NO3 | [MDL Number]
MFCD00067306 | [Molecular Weight]
289.37 | [MOL File]
101-31-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
108,5°C | [alpha ]
D20 -21.0° (alc) | [Boiling point ]
431.53°C (rough estimate) | [density ]
1.0470 (rough estimate) | [refractive index ]
1.5200 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO:79.0(Max Conc. mg/mL);273.0(Max Conc. mM)
Ethanol:58.0(Max Conc. mg/mL);200.43(Max Conc. mM) | [form ]
powder
| [pka]
pKa (21°) 9.7 | [color ]
white
| [biological source]
rabbit | [Water Solubility ]
3.6g/L(20 ºC) | [Merck ]
4858 | [InChIKey]
RKUNBYITZUJHSG-FXUDXRNXSA-N | [LogP]
1.380 (est) | [CAS DataBase Reference]
101-31-5(CAS DataBase Reference) | [NIST Chemistry Reference]
Hyoscyamine(101-31-5) | [EPA Substance Registry System]
101-31-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R26/28:Very Toxic by inhalation and if swallowed . | [Safety Statements ]
S24:Avoid contact with skin .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 1544 6.1/PG 2
| [WGK Germany ]
3
| [RTECS ]
NH0875000
| [HazardClass ]
6.1 | [PackingGroup ]
I | [HS Code ]
29339900 | [Hazardous Substances Data]
101-31-5(Hazardous Substances Data) | [Toxicity]
man,LDLo,unreported,1471ug/kg (1.471mg/kg),"Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. |
| Hazard Information | Back Directory | [Chemical Properties]
White to Off-White Solid | [Uses]
Suggested starting dilutions are as follows: IHC-P: 1:100-1:1000, WB: 1:1000-1:10000. Not yet tested in other applications. Optimal working dilutions should be determined experimentally by the end user. | [Uses]
L-Hyoscyamine can be used as anticholinergic and analgesic.
| [Uses]
L-Hyoscyamine is a natural compound that has inhibitory activity against cholinesterases.
| [Definition]
ChEBI: An atropine with a 2S-configuration. | [General Description]
Hyoscyamine is a levorotatory alkaloidobtained from various solanaceous species. One ofthe commercial sources is Egyptian henbane (Hyoscyamusmuticus), in which it occurs to the extent of about 0.5%.Usually, it is prepared from the crude drug in a manner similarto that used for atropine and is purified as the oxalate.The free base is obtained easily from this salt.
It occurs as white needles that are sparingly soluble inwater (1:281), more soluble in ether (1:69) or benzene(1:150), very soluble in chloroform (1:1), and freely solublein alcohol. It is used as the sulfate and hydrobromide. Theprincipal reason for the popularity of the hydrobromide hasbeen its nondeliquescent nature. The salts have the advantageover the free base in being quite water soluble. | [Biochem/physiol Actions]
The 21st amino acid, selenocysteine (sec), is distinct from other amino acids because it lacks its own tRNA synthetase and is the only amino acid synthesized on its cognate tRNA. Synthesis of sec begins with acylation of tRNA(sec) (TRSP; MIM 165060) by seryl-tRNA synthetase (SARS; MIM 607529) to give ser-tRNA(sec), which is subsequently phosphorylated by O-phosphoseryl-tRNA kinase (PSTK; MIM 611310) to give O-phosphoseryl-tRNA(sec). SEPSECS catalyzes the final step of sec synthesis by converting O-phosphoseryl-tRNA(sec) to selenocysteinyl-tRNA(sec) using selenophosphate as the selenium donor (Palioura et al., 2009 [PubMed 19608919]).[supplied by OMIM] | [Clinical Use]
(L-Hyoscyamine)Hyoscyamine is used to treat disorders of the urinarytract more so than any other antispasmodic, although thereis no evidence that it has any advantages over the otherbelladonna preparations and the synthetic anticholinergics.It is used to treat spasms of the bladder and, in this manner,serves as a urinary stimulant. It is used together witha narcotic to counteract the spasm produced by the narcoticwhen the latter is used to relieve the pain of urethral colic.Hyoscyamine preparations are also used as antispasmodicsin the therapy of peptic ulcers.
| [target]
AChR |
|
|