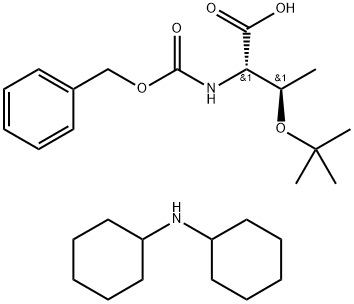
Z-THR(TBU)-OH DCHA synthesis
- Product Name:Z-THR(TBU)-OH DCHA
- CAS Number:16966-07-7
- Molecular formula:C28H46N2O5
- Molecular Weight:490.69
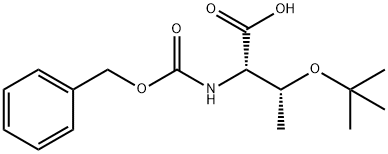
16966-09-9
53 suppliers
$65.00/25 g
101-83-7
433 suppliers
$10.00/25g

16966-07-7
135 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
in methanol at 0; for 0.333333 h;
Steps:
DCA salt formation of AA
General procedure: To a solution of PHN-AA-OH (1.00 equiv.) in MeOH (3.50 mL)at 0 °C was added dicyclohexylamine (DCA) (1.10 equiv.). After 20 min, the white suspensionformed was filtered out over vacuum and the solids were washed out with EtOAc (3x 5 mL).The PHN-AA-OH.DCHA salts were isolated as white solids with yields ranging from 92-95%.DCA salts were prepared for Cbz-Ala-OH and Cbz-Thr(tBu)OH amino acids (AA).
References:
Suppo, Jean-Simon;De Sant'Ana, Danilo Pereira;Dias, Luiz Carlos;De Figueiredo, Renata Marcia;Campagne, Jean-Marc [Synthesis,2014,vol. 46,# 22,art. no. SS-2014-T0388-OP,p. 3075 - 3084] Location in patent:supporting information