
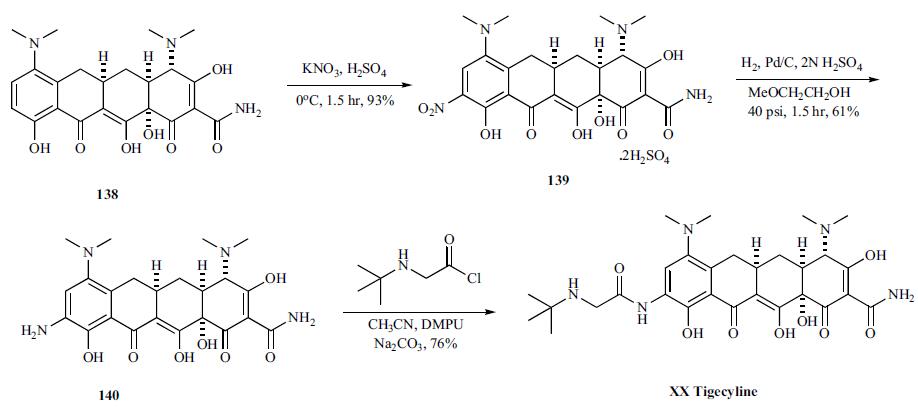
Tigecycline synthesis
- Product Name:Tigecycline
- CAS Number:220620-09-7
- Molecular formula:C29H39N5O8
- Molecular Weight:585.65
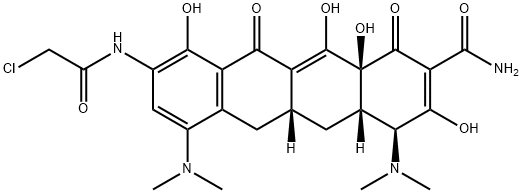
156263-28-4
4 suppliers
inquiry
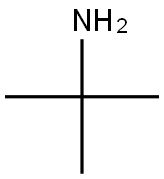
75-64-9
441 suppliers
$10.00/10g

220620-09-7
510 suppliers
$7.00/10mg
Yield:220620-09-7 92%
Reaction Conditions:
with sodium iodide in N,N-dimethyl acetamide at 50; for 2 h;
Steps:
1
25 ml N,N-dimethylacetamide and 10.0 g 9-chloroacetaminominocycline x 1.5 TBA (t- butylamine) were put into a three-necked- flask at room temperature. 9.05 g t-butylamine and 3.0 g sodium iodide were added to the suspension and the reaction mixture was stirred for 2 hours at 50 °C. Then the reaction mixture was transferred into a Schmizo reactor and diluted with 250 ml methylene chloride and 250 ml water. The pH was adjusted to 8.29 (+/- 0.1 ) by dropwise adding concentrated hydrochloric acid. After stirring the mixture for 5 to 10 minutes the layers were separated. Before the aqueous layer was washed two times with 250 ml methylene chloride, the pH was adjusted to 8.0 +/- 0.1 with 0.1 N sodium hydroxide. The united organic layers were washed three times with 250 ml water and again the pH was adjusted to 8.0 by dropwise adding either 0.1 N hydrochloric acid or 0.1 N sodium hydroxide before washing. Then the organic layer was filtered through a fluted filter and evaporated at 40 °C on the rotavapor. The liquid residue was diluted with 100 ml of methylene chloride and again transferred into a Schmizo reactor. Tigecycline was crystallized within a few minutes by the addition of 120 ml n-heptane. The suspension was stirred for 3 hours at room temperature and over night at 5 °C. The solid was filtered off, washed with a cold methylene chloride/ n-heptane (3:7) mixture and dried under vacuum over night to obtain 8.2 g (92 % of
References:
WO2009/92680,2009,A2 Location in patent:Page/Page column 7
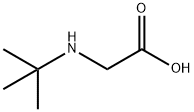
58482-93-2
54 suppliers
inquiry
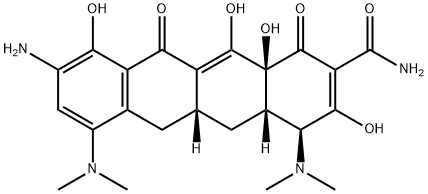
149934-19-0
27 suppliers
inquiry

220620-09-7
510 suppliers
$7.00/10mg