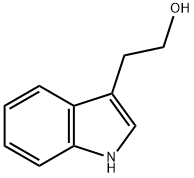
Tryptophol synthesis
- Product Name:Tryptophol
- CAS Number:526-55-6
- Molecular formula:C10H11NO
- Molecular Weight:161.2
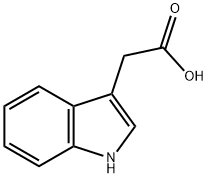
87-51-4
729 suppliers
$11.19/5G

526-55-6
325 suppliers
$5.00/50mg
Yield:526-55-6 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran for 3 h;Reflux;
Steps:
Intermediate 302-(1 H-inclol-3-yl) ethanol
To a solution of 3-indoleacetic acid (ig, 5.7mmol) in THE (30m1) was added a IM solution of lithium aluminium hydride in THE (11.4m1, 11.4mmol) and the mixture refluxed for 3 hours. The mixture was cooled, 0.43m1 of water carefully added, followedby 0.43ml of 15% NaOH(ag) and finally 1 ,5ml of water. The solids were filtered from the mixture, washed with EtOAc and the filtrate concentrated to give 2-(1 H-indol-3- yl)ethanol (919mg, 100%); 1H NMR (400 MHz, CHLOROFORM-d) 6 ppm 3.06 (t, J=6.40 Hz, 2 H), 3.93 (t, J=6.40 Hz, 2 H), 7.10 (d, J-2.29 Hz, 1 H), 7.12 -7.18 (m, 1 H), 7.20-7.26 (m, I H), 7.36-7.41 (m, 1 H), 7.64 (dd, J=8.01, 1.14 Hz, I H), 8.10 (br. 5., 1H).
References:
MEDICAL RESEARCH COUNCIL TECHNOLOGY;WINTER-HOLT, Jon James;MCIVER, Edward Giles;AMBLER, Martin;LEWIS, Stephen;OSBORNE, Joanne;WEBB-SMITH, Kayleigh WO2017/85484, 2017, A1 Location in patent:Page/Page column 67; 68
1191-99-7
342 suppliers
$10.00/10g
59-88-1
374 suppliers
$15.00/10g

526-55-6
325 suppliers
$5.00/50mg
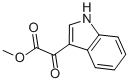
18372-22-0
155 suppliers
$8.00/250mg

526-55-6
325 suppliers
$5.00/50mg
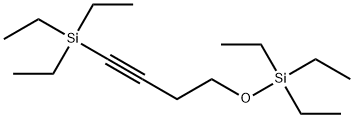
160194-28-5
27 suppliers
$60.00/5g
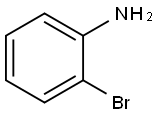
615-36-1
421 suppliers
$10.00/5g

526-55-6
325 suppliers
$5.00/50mg
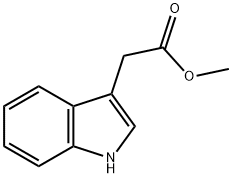
1912-33-0
152 suppliers
$8.00/1g

526-55-6
325 suppliers
$5.00/50mg