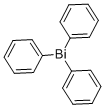
Triphenylbismuth synthesis
- Product Name:Triphenylbismuth
- CAS Number:603-33-8
- Molecular formula:C18H15Bi
- Molecular Weight:440.29
Yield:603-33-8 86%
Reaction Conditions:
Stage #1:bromobenzene with lithium in diethyl ether for 0.5 h;Reflux;
Stage #2:bismuth(III) chloride in diethyl ether at 20; for 15 h;
Steps:
3.B B) Preparation of Triphenylbismuthane, Ph3Bi
5.52 g (795 mmol) of 20 lithium in 250 ml of 14 diethyl ether (Et2O) are initially introduced in a 1 l three-necked flask with reflux condenser. 44 ml (420 mmol) of 21 bromobenzene are slowly added dropwise via a 250 ml dropping funnel with stirring, so that the diethyl ether boils continuously. When the addition is complete, the grey-brown suspension is heated under reflux for 30 min, and 32.74 g (104 mmol) of 22 bismuth trichloride are subsequently added. The mixture is stirred at RT for 15 h. The suspension is hydrolysed using distilled 23 water with ice-bath cooling and neutralised using a saturated 24 ammonium chloride solution. The organic phase is separated off, and the aqueous phase is extracted twice with Et2O. The combined organic phases are dried over MgSO4, and the solvent is removed in a high vacuum. The residue is recrystallised from 80 ml of Et2O. Repeated decantation and evaporation of the mother liquor and drying in a high vacuum gives 9 triphenylbismuthane in the form of colourless needles. The yield is 39.26 g (89.21 mmol, 86% based on bismuth trichloride). 1H NMR (CDCl3), δ, ppm: 7.8 (m, Hortho), 7.4 (m, Hmeta, Hpara).
References:
MERCK PATENT GMBH;HOGE, Berthold Theo;SOLYNTJES, Sven Joerg-Ruediger August;IGNATIEV, Nikolai (Mykola) US2018/273561, 2018, A1 Location in patent:Paragraph 0088; 0089; 0090; 0091

7787-60-2
388 suppliers
$6.00/25g
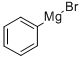
100-58-3
196 suppliers
$18.00/10ml

603-33-8
267 suppliers
$29.69/5g
